SeromYx Systems Announces Strategic Collaboration with University of Pittsburgh to Advance CAR-T Therapy for Solid Tumors
SeromYx Systems, inc. a global leader in antibody functional profiling services and Systems Serology, is proud to announce a research collaboration with the lab of Dr. Jason Lohmueller, a synthetic biologist at the University of Pittsburgh, aimed at advancing the development of CAR-T therapies for solid tumors.
While CAR-T cell therapy has shown remarkable success in achieving durable remissions in hematologic malignancies, the broader application of this approach including treatment of solid tumors faces substantial limitations due to challenges such as therapeutic resistance from antigen heterogeneity and toxicities.
The Lohmueller lab, located at the UPMC Hillman Cancer Center, a National Cancer Institute designated Comprehensive Cancer Center, is at the forefront of addressing these obstacles through innovative synthetic biology approaches. His team has pioneered the development of “universal” SNAP adaptor CAR and synthetic Notch (synNotch) T cells, offering a modular and programmable platform for more precise and effective targeting of multiple solid tumor antigens with co-administered engineered antibodies (Ruffo et al., Nat Commun, 2023). These cutting-edge technologies promise to overcome many of the barriers that have historically limited the success of adoptive T cell therapy in solid tumors and are licensed for clinical development by Coeptis Therapeutics.
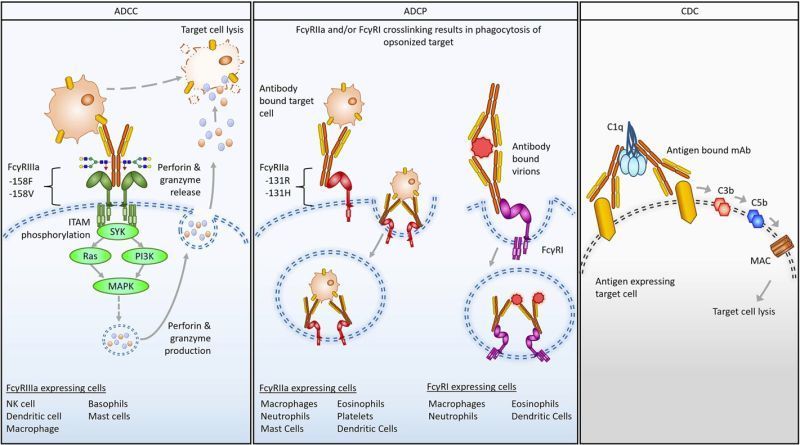
As part of this collaboration, SeromYx is conducting comprehensive biophysical and cellular Fc function profiling of several engineered adaptor antibodies directed against clinically validated solid tumor targets. The company’s Systems Serology platform is being leveraged to evaluate critical immune effector functions and identify optimal antibody-CAR adaptor configurations.
“This collaboration marks an exciting step forward in the pursuit of more effective and accessible CAR-T therapies,” said Lenny Moise, VP, Research at SeromYx Systems. “Dr. Lohmueller’s groundbreaking work in synthetic receptor design is opening entirely new avenues for modular and tunable T cell therapies. By combining our deep expertise in immune profiling with his innovative CAR-T constructs, we aim to generate critical insights that can accelerate the development of next-generation treatments for solid tumors.”
Dr. Lohmueller added, “SeromYx’s cutting-edge immune profiling platform and antibody expertise has already yielded valuable insights into the immunological functions of our adaptor candidates beyond their CAR T activity. These findings are key to guiding our development efforts, and it has been fantastic working with the SeromYx team.”
This partnership exemplifies SeromYx Systems’ ongoing commitment to applying its advanced Systems Serology technologies to enable breakthroughs in translational immunology and the company anticipates that this joint research will contribute to meaningful advances in the field of cancer immunotherapy.
For more information about SeromYx Systems and its pioneering work in Systems Serology services, visit https://www.seromyx.com/.
About SeromYx Systems:
SeromYx Systems is a global leader in providing high-throughput antibody characterization services and Systems Serology, offering advanced solutions for the comprehensive analysis of immune responses. With a dedication to innovation and excellence, and GCLP-accreditation, SeromYx is at the forefront of revolutionizing the fields of translational and clinical immunology through its cutting-edge technologies and strategic collaborations.
Contact:
Abigail Harris
Business Development Manager
abigail.harris@seromyx.com