Impact of antibody Fc engineering on translational pharmacology, and safety: insights from industry case studies
The Fc Review:
Continuing our series taking a closer look at recent Fc-focused papers, what they found, and why it matters for antibody discovery and development.
How does Fc engineering shape the translation of antibodies from preclinical models to the clinic?
A new industry-wide review with 15 case studies examines the impact of Fc modifications on pharmacology and safety, and the challenges of predicting human outcomes from nonclinical studies.
Background:
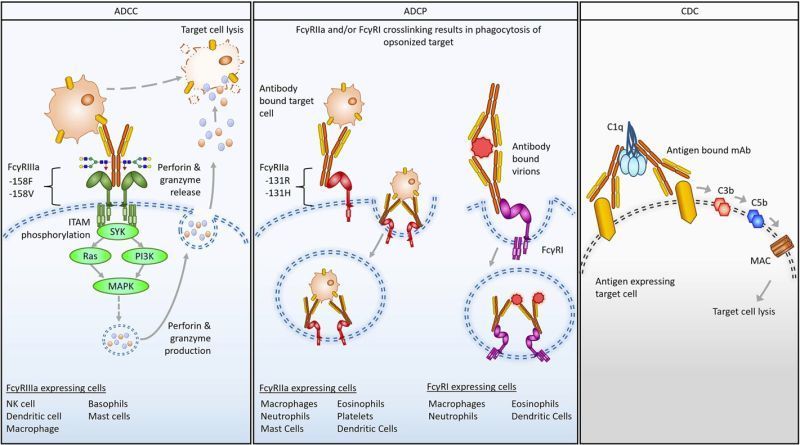
Fc regions do not only extend half-life, they drive functions like ADCC, ADCP, CDC, and immune modulation. Engineering the Fc can enhance, silence, or redirect these activities. But the same changes that deliver potency can also introduce risk, especially when preclinical models do not fully mirror human Fc receptor biology.
The review highlights:
- Species differences in FcγR and FcRn expression complicate translation from rodent and NHP models to humans.
- Afucosylation and amino acid substitutions can strongly boost effector functions but may also uncover safety liabilities.
- Fc silencing and half-life extension strategies require careful assessment to avoid unintended biology.
- Case studies underscore the need for multi-pronged evaluation that combines specialized in vitro human primary-cell assays, transgenic models, and orthogonal biophysical methods such as SPR.
Implications:
The paper makes clear that Fc engineering is powerful, but its impact is context dependent. Translating nonclinical data requires assays that capture human biology and anticipate both efficacy and safety outcomes.
Our perspective:
Fc engineering can deliver meaningful gains, but each modification can ripple across multiple pathways from effector function to safety. That is why broad profiling across FcγR interactions, complement pathways, and primary-cell functions is essential. Evaluating engineered antibodies in a wide panel of human-relevant functional and biophysical assays, which form the core of SeromYx' assay portfolio, provides the clearest view of both intended effects and unanticipated trade offs.