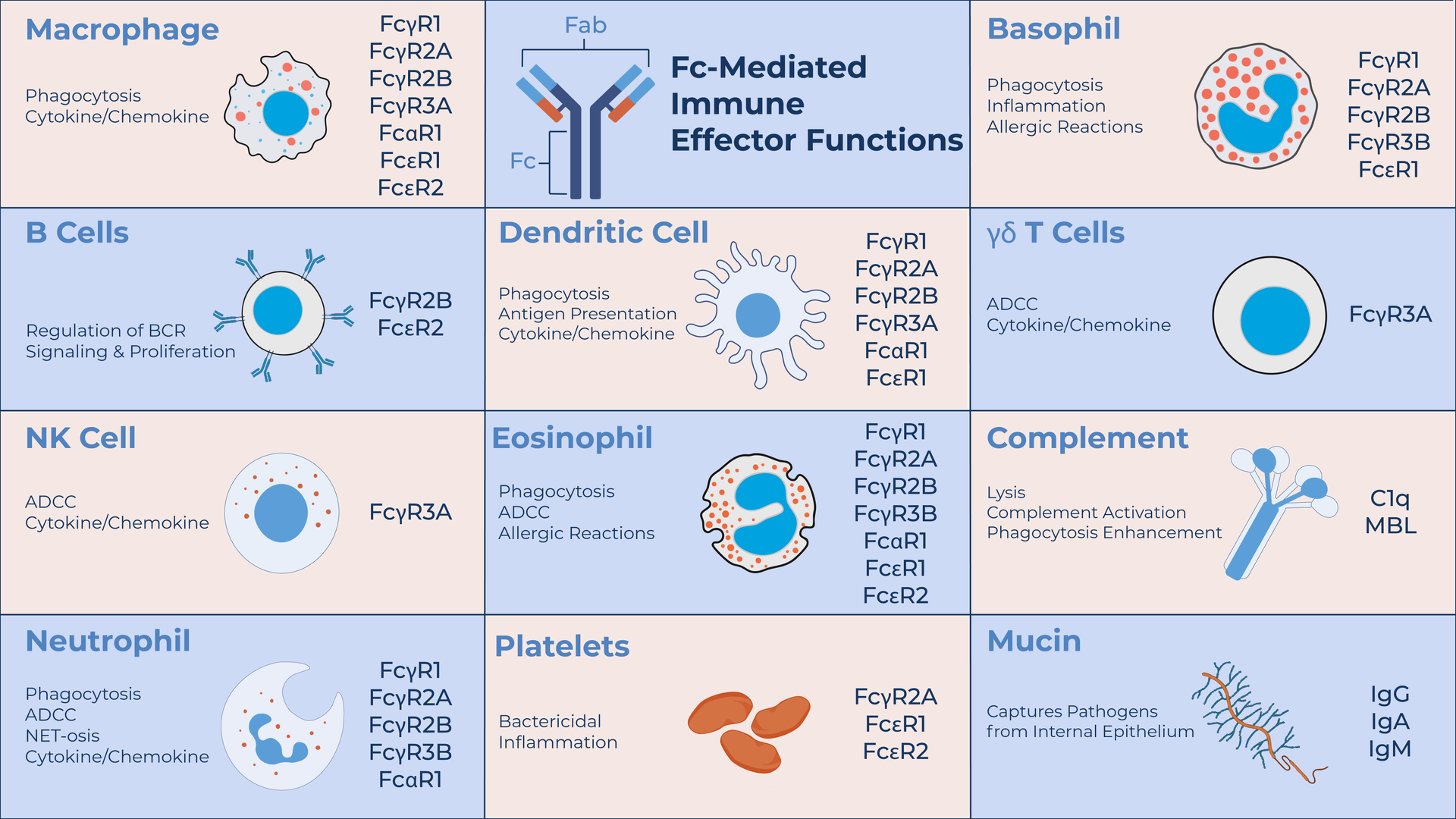
SeromYx's technology combines a broad suite of biophysical and functional assays to characterize the diverse Fc-mediated effector functions of antibodies.
Using primary immune cells, our platform delivers a detailed view of immune activity and mechanism, helping teams connect Fc structure with function to guide development decisions.
ASSAY CAPABILITIES
SeromYx offers one of the most extensive panels of Fc effector function assays available, spanning biophysical interaction profiling and cell-based functional assays.
Assays are performed in
96–384-well formats
and can scale efficiently to thousands of samples per run.
Assays are available for
human, non-human primate, and murine
systems, and can be adapted to other species upon request.
BIOPHYSICAL ASSAYS
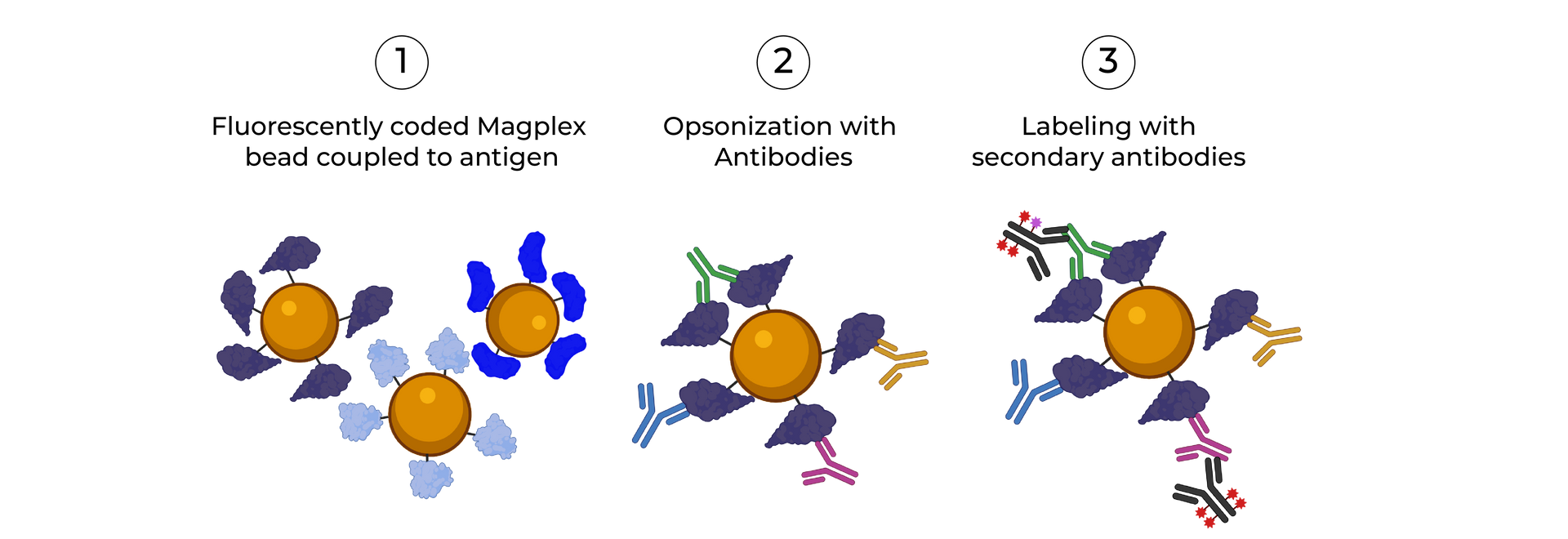
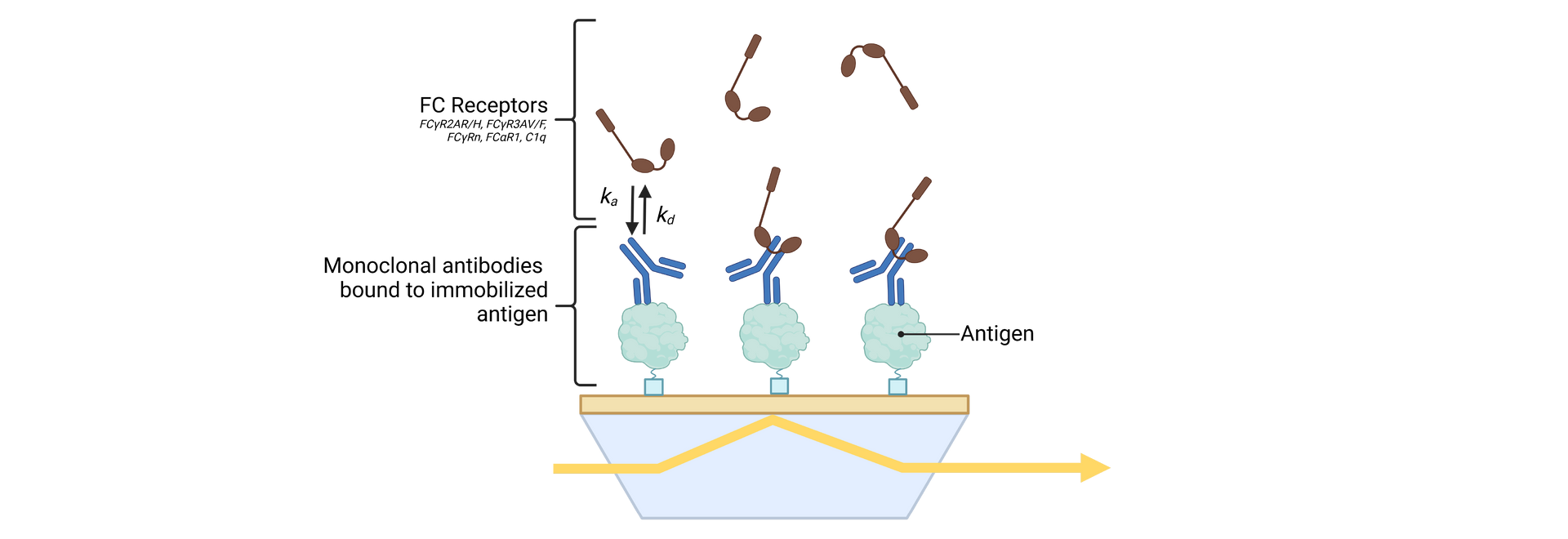
Fc Receptor Binding Array
Fluorescently coded microspheres capture multiple antigen specificities simultaneously and profile the effector capacity by assessing interaction of antigen-specific antibodies with Fc receptors.
Available receptors: FCGR2A (R131, H131), FCGR2B, FCGR3A (V158, F158), FCGR3B, FCRN (pH 6.0, 7.4), FCA, C1q, TRIM21.
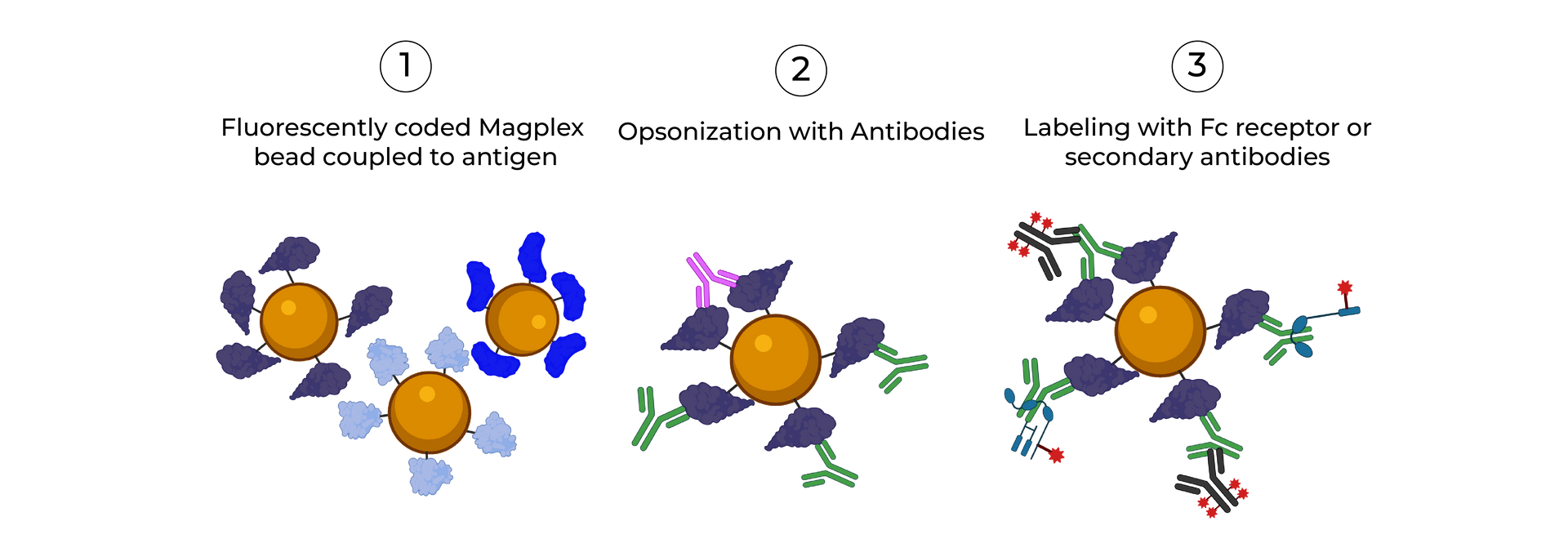
Antigen Specific Antibody Isotyping and Subclassing (ISSC)
Fluorescently coded microspheres capture multiple antigen specificities simultaneously and profile the isotype/subclass distribution in an antigen-specific manner.
Available detectors: Total IgG, IgG1, IgG2, IgG3, IgG4, IgA1, IgA2, IgM, IgE.
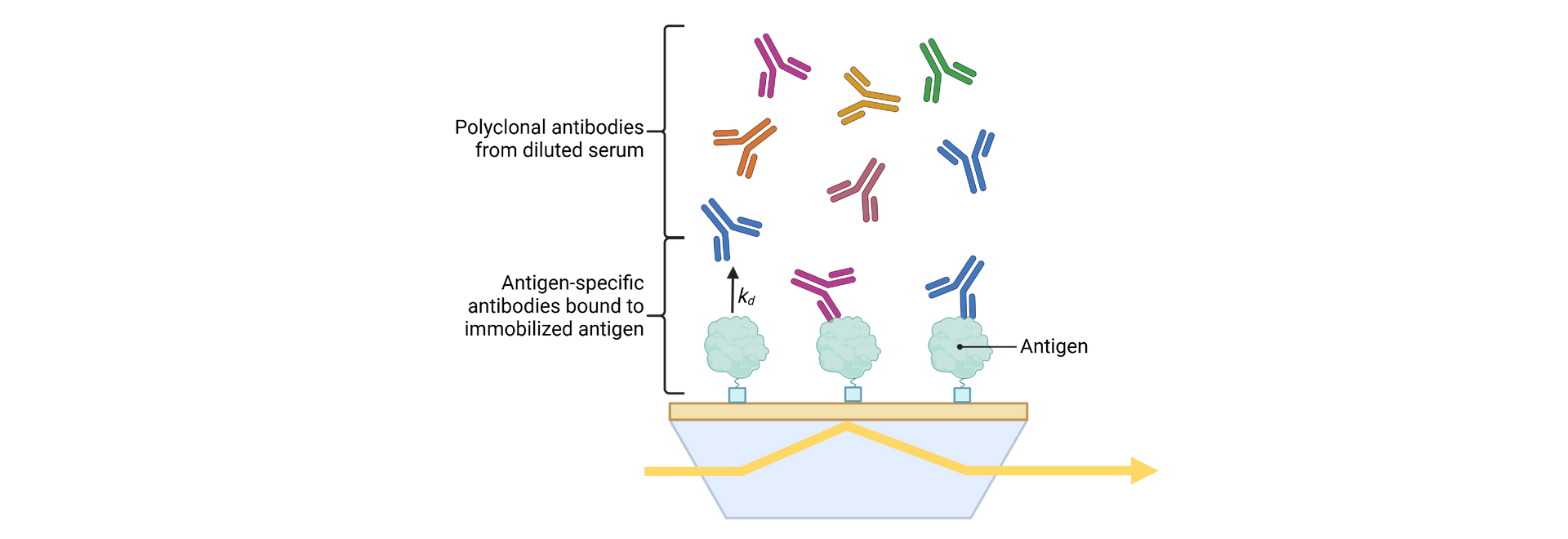
Antibody Avidity by SPR
Avidity Index measures the strength of binding to a target antigen by either polyclonal antibodies from serum or monoclonal antibody cocktails. The off-rate is utilized along with the magnitude of response to compute avidity index.
Antibody Affinity by SPR
Tripartite binding measures traditional antibody affinity kinetics in an antigen-specific manner. The kinetics of the antigen-antibody-receptor interaction describe the on-rate, the off-rate, and the associated affinity (equilibrium dissociation constant, KD) of the antibody with the receptor. We also conduct bipartite Fc-FcR affinity measurements.
CELLULAR FUNCTIONAL ASSAYS
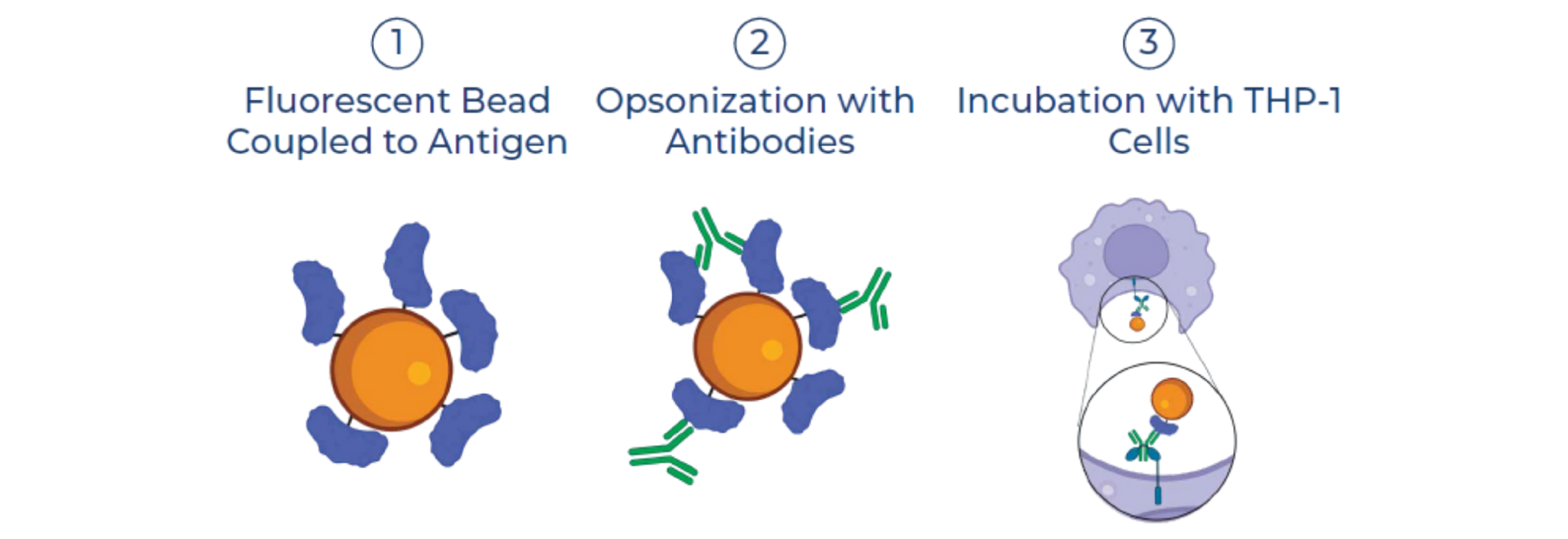
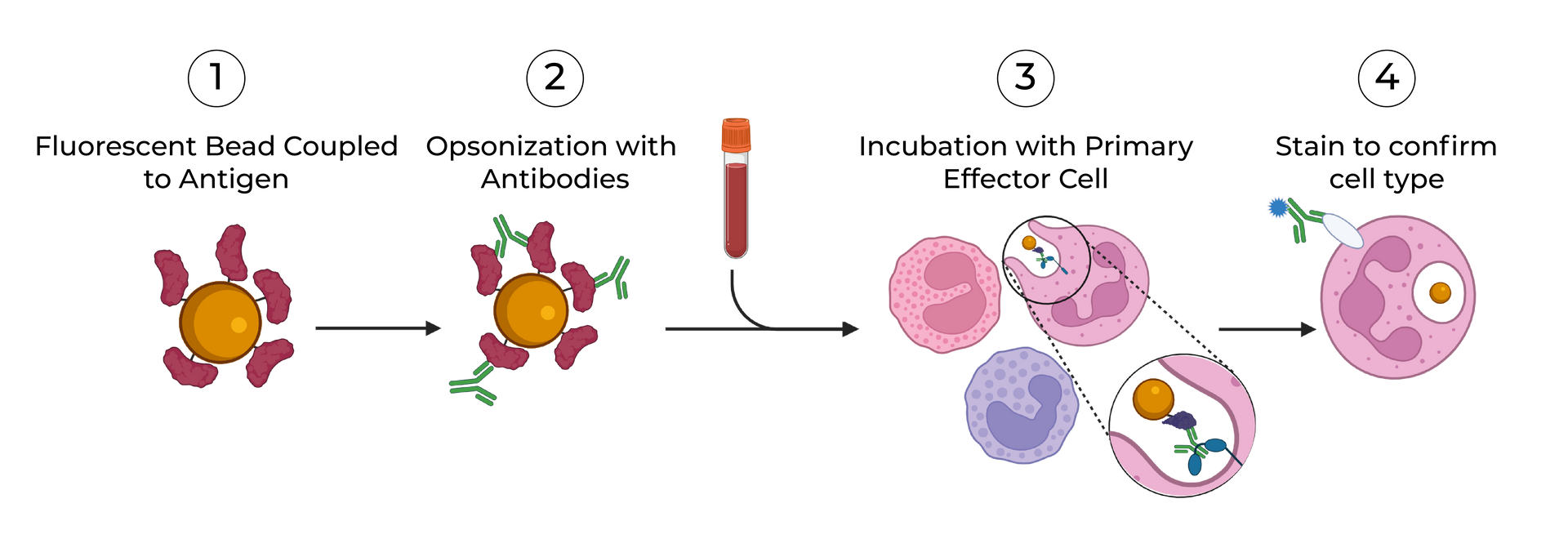
Antibody Dependent Cellular Phagocytosis (ADCP)
Assesses the ability of antibodies to induce phagocytosis of antigen- functionalized fluorescent beads by monocytes via Fc receptors.
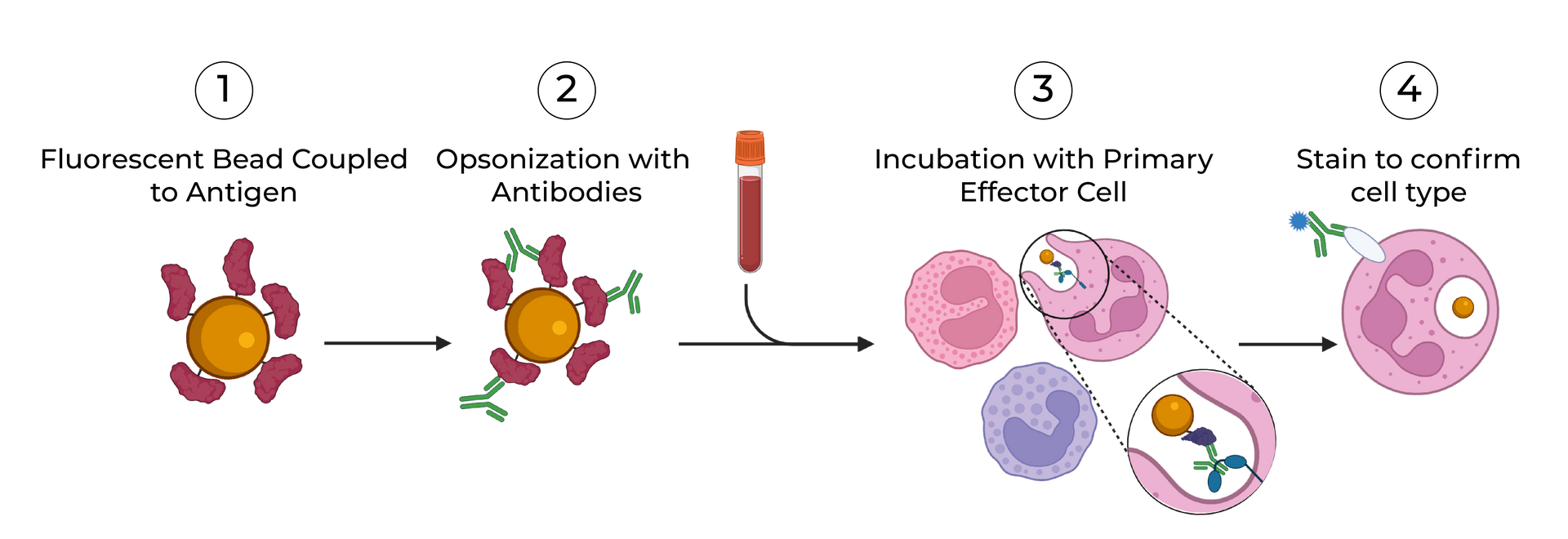
Antibody Dependent Neutrophil Phagocytosis (ADNP)
Assesses the ability of antibodies to induce the phagocytosis of antigen-coated targets by primary neutrophils.
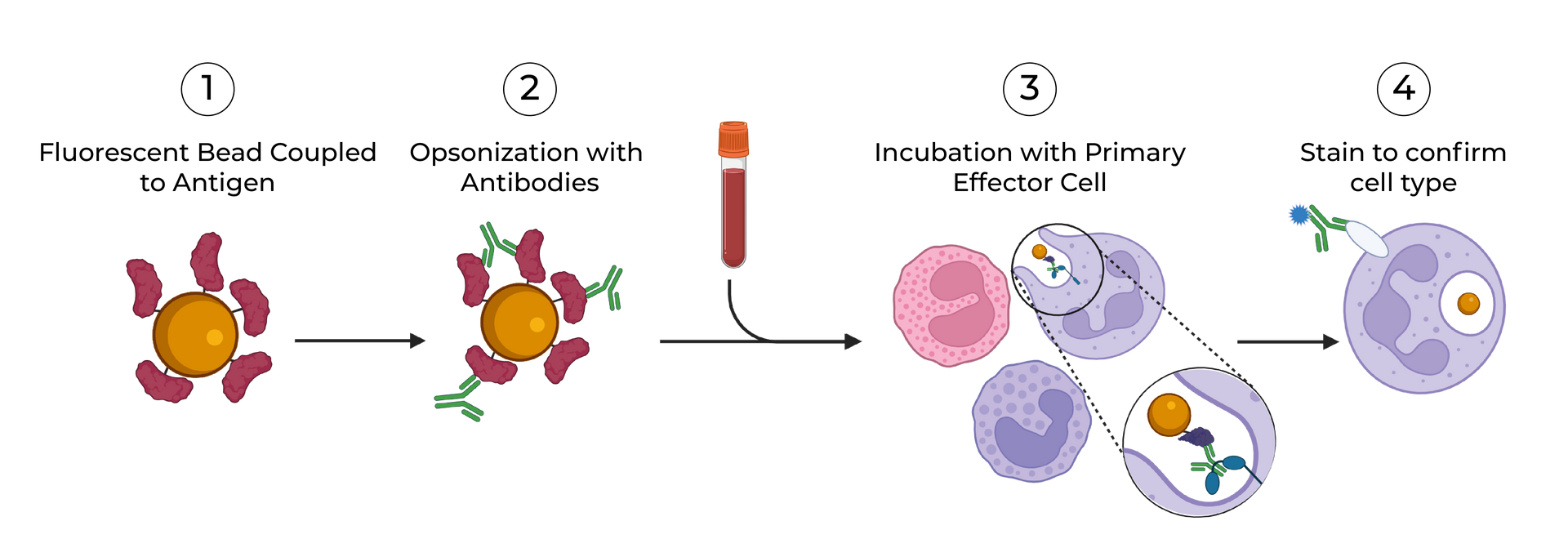
Antibody Dependent Basophil Phagocytosis (ADBP)
Assesses the ability of antibodies to induce the phagocytosis of antigen-coated targets by primary basophils.
Antibody Dependent Eosinophil Phagocytosis (ADEP)
Assesses the ability of antibodies to induce the phagocytosis of antigen-coated targets by primary eosinophils.
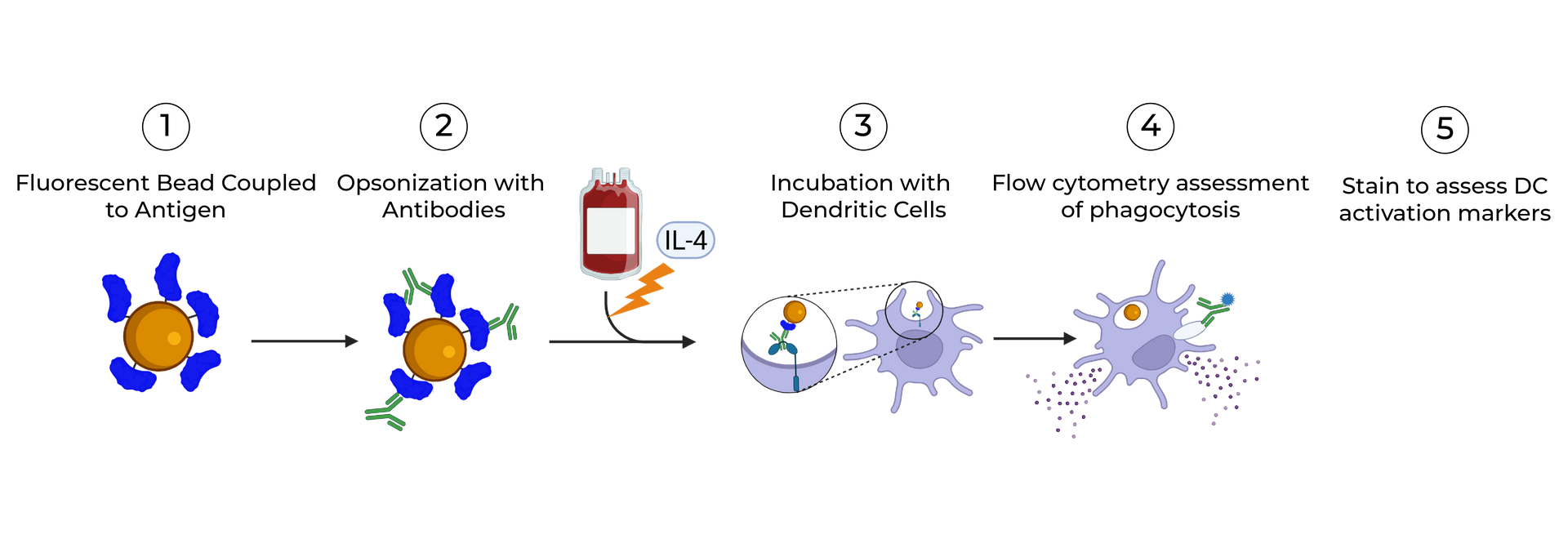
Assesses the ability of antibodies to induce phagocytosis of antigen-coated targets by dendritic cells as well as DC activation/maturation and cytokine release.
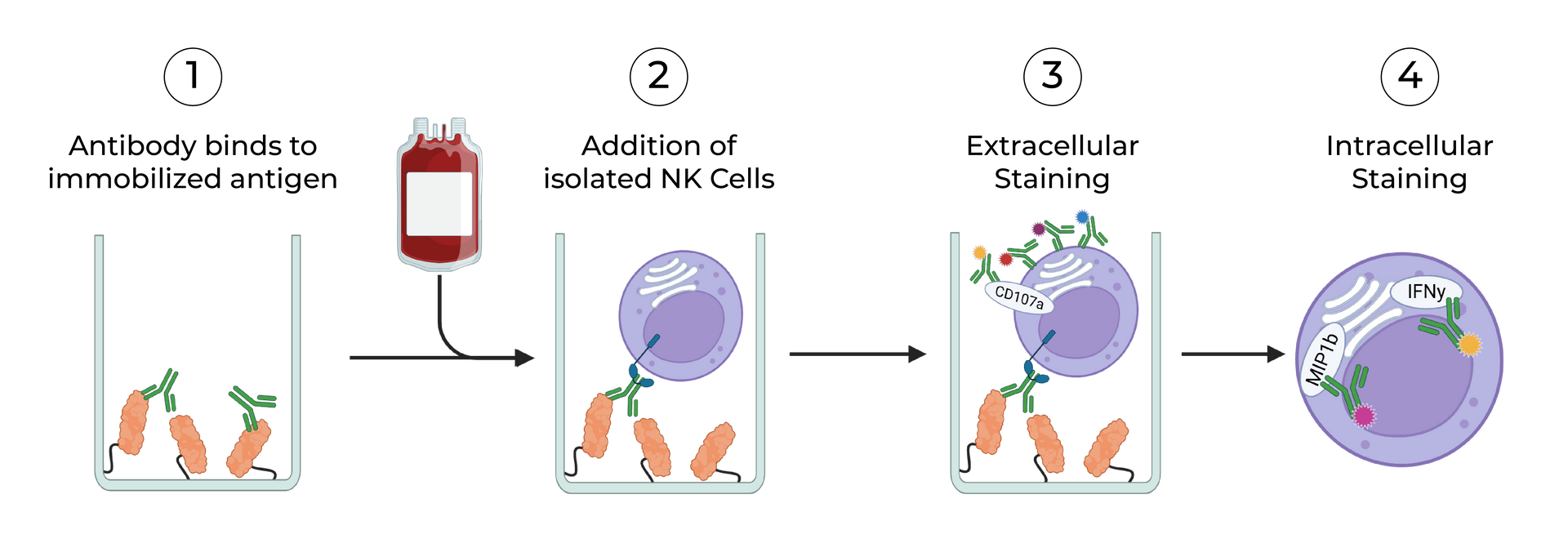
Antibody Dependent NK Cell
Activation (ADNKA)
Assesses the ability of antibodies to induce NK cell activation against antigen-coated plates by measuring the levels of CD107a, IFN-γ and MIP-1β.
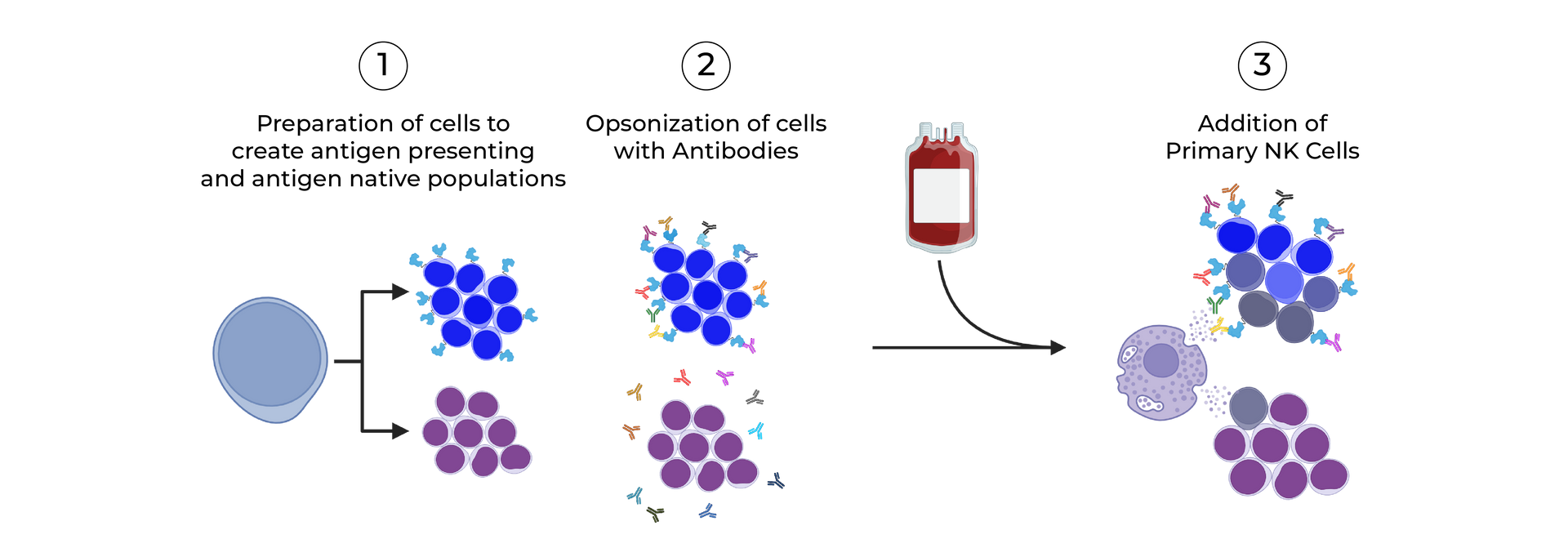
Antibody Dependent Cellular
Cytotoxicity (ADCC)
Tests the ability of antigen-specific antibodies to recruit NK cell lytic activity.
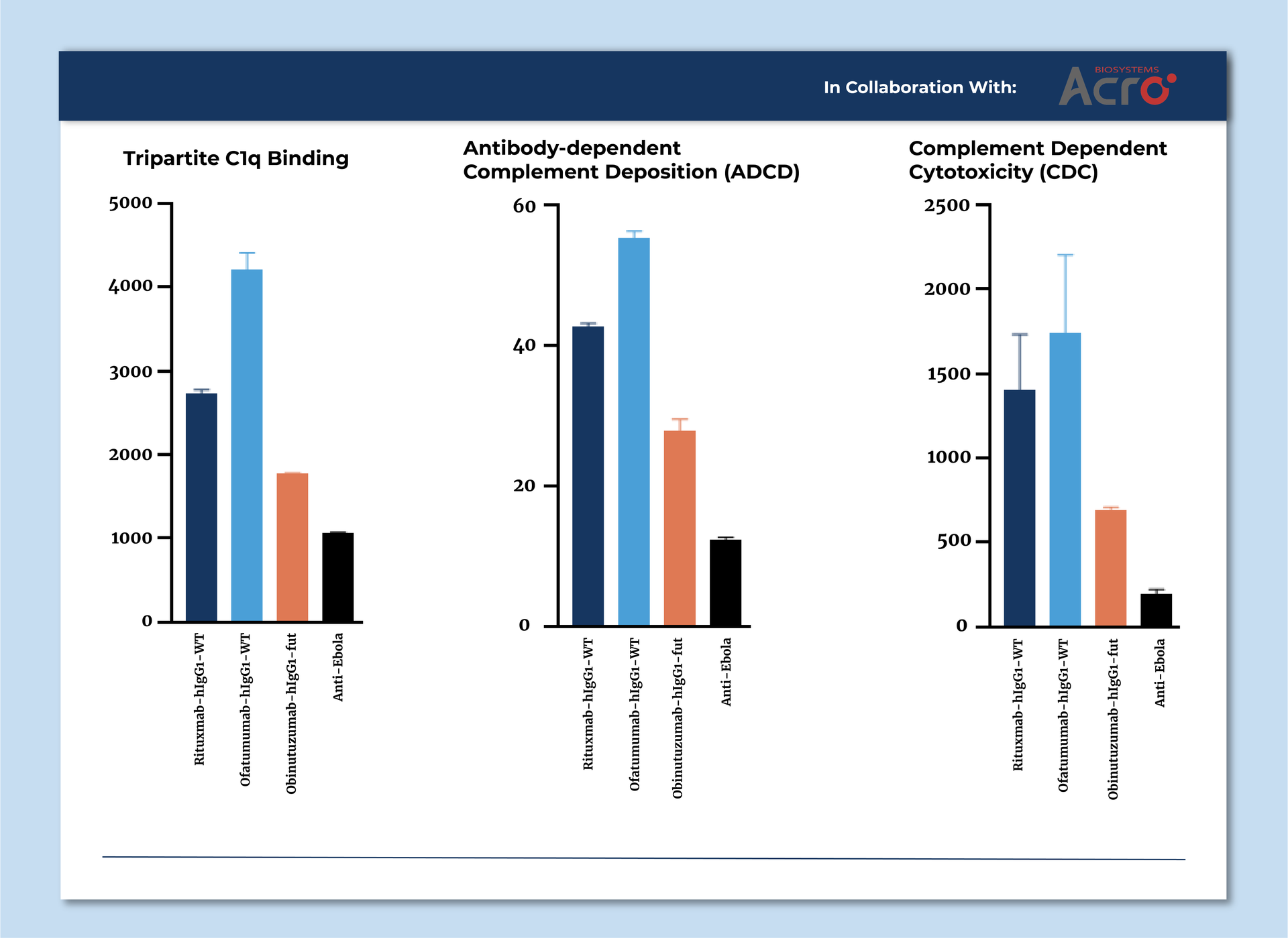
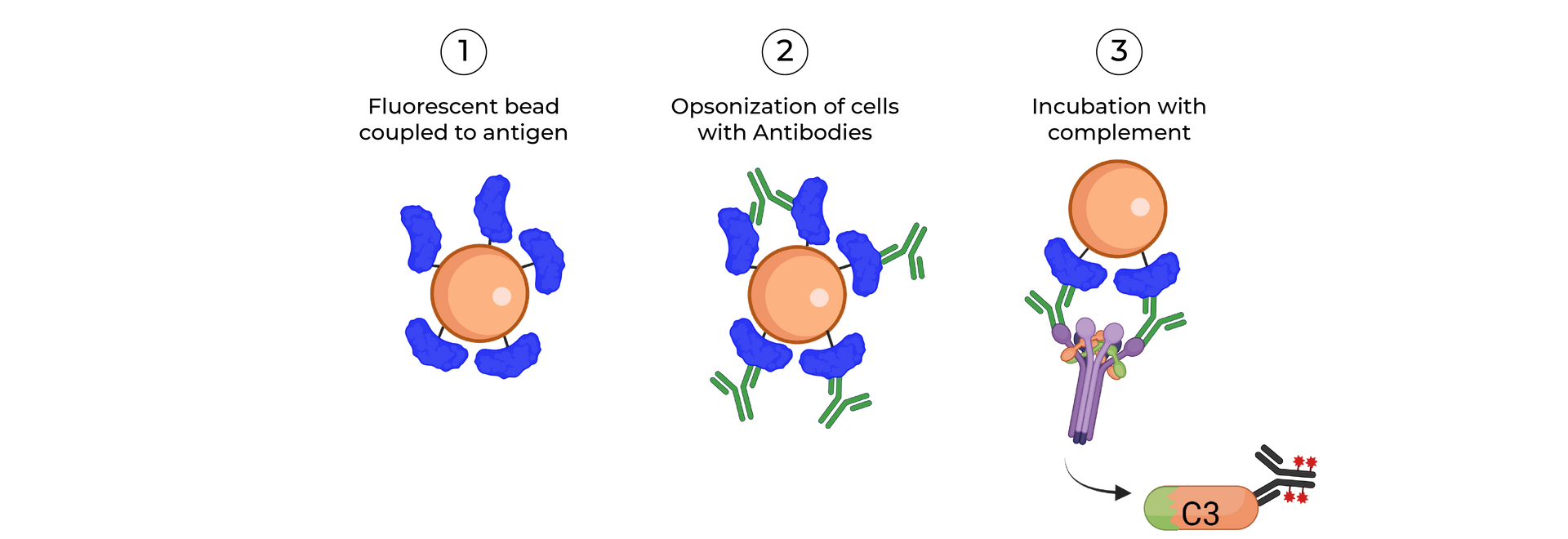
Antibody Dependent Complement Deposition (ADCD)
Assesses the recruitment of complement component C3b on the surface of antigen-coupled beads.
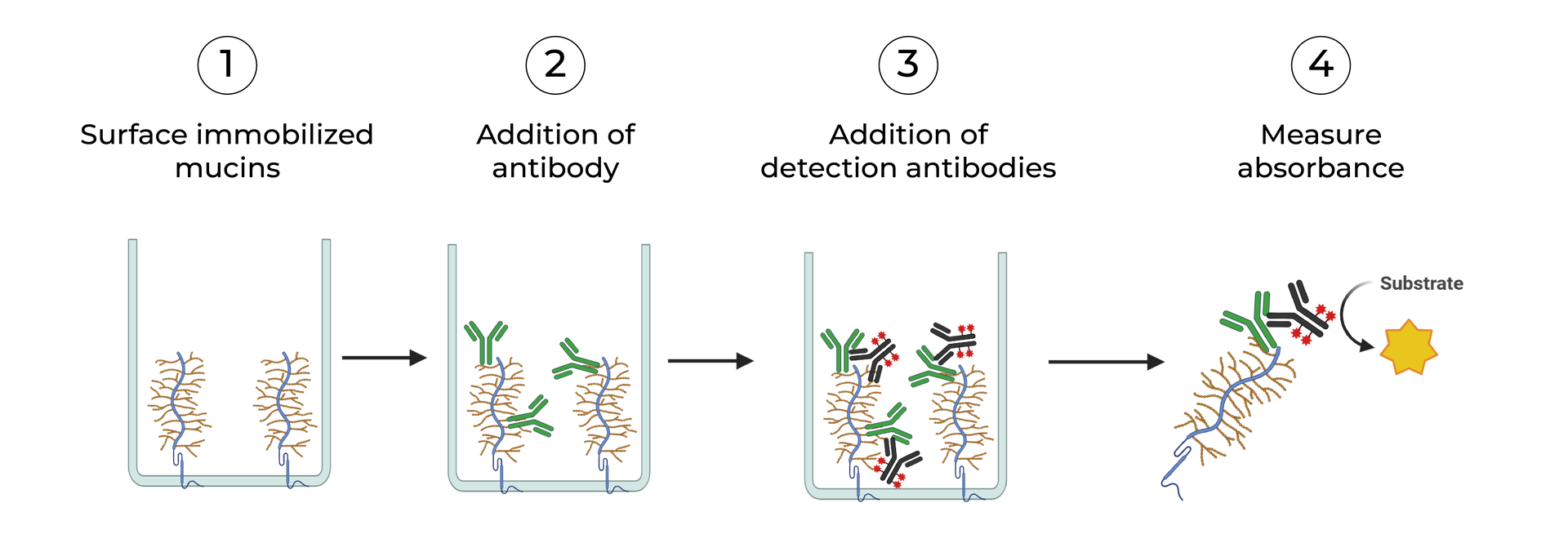
Antibody Dependent Mucin
Binding (ADMB)
Measures the capacity of antibodies to trap pathogens in mucus proteins.
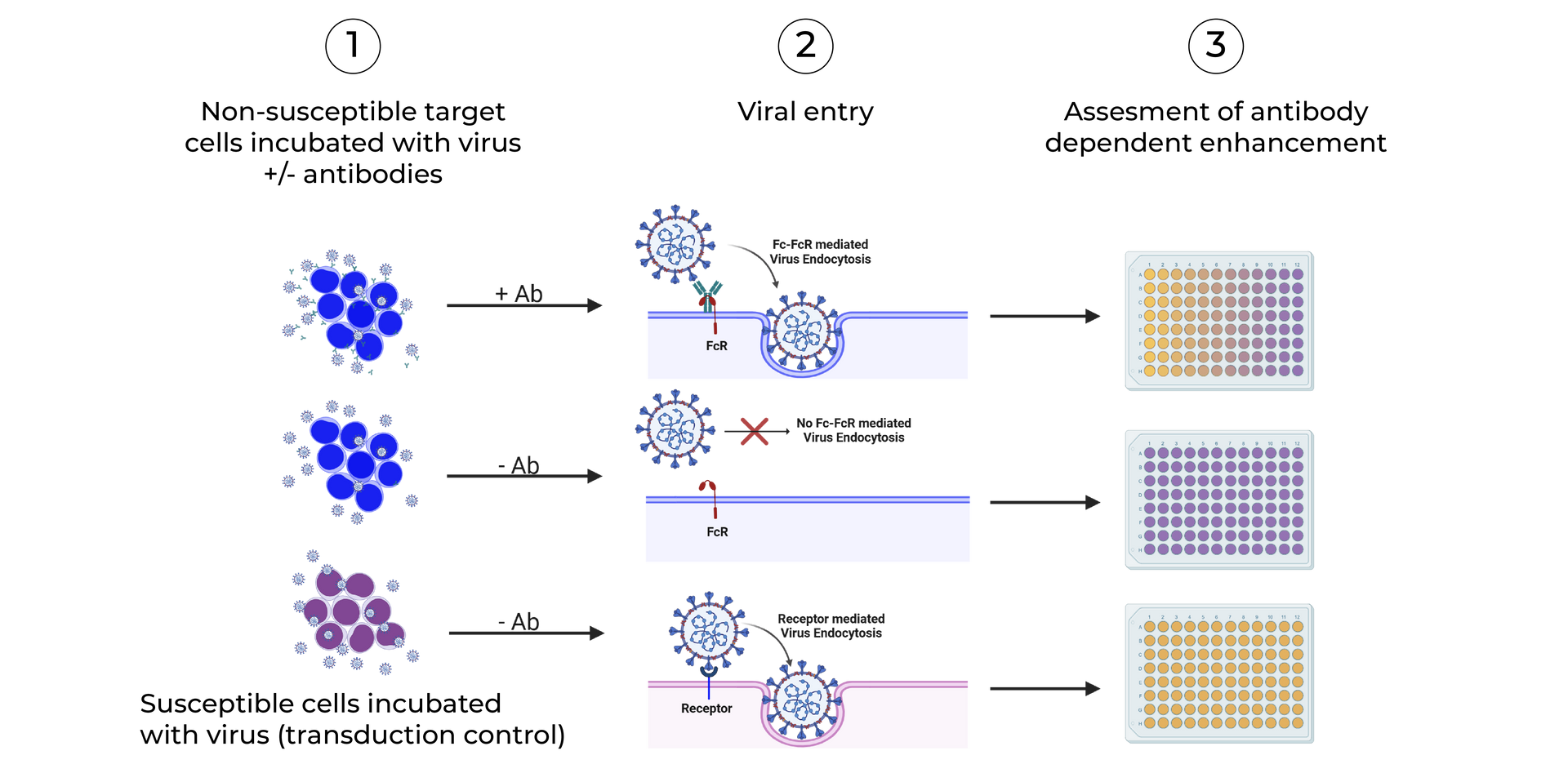
Antibody Dependent Enhancement
of Infection (ADEI)
Measures the enhancement of viral infection caused by antigen specific antibodies in non-susceptible target cells.