The Fc Review series highlights recent scientific advances in Fc biology and their impact on antibody discovery and development. Each post breaks down key findings and why they matter, with perspective from SeromYx on how to translate them into better therapeutic decision-making.

The Fc Review: How are antibody developers actually using Fc engineering today? A recent analysis of the IMGT/mAb-DB database takes a systematic look at engineered Fc variants across therapeutic antibodies and fusion proteins, offering a real-world snapshot of how Fc design choices are being deployed in the clinic. Background: Fc engineering is often discussed in terms of individual mutations or isolated use cases. But at an industry level, it’s less clear how frequently Fc variants are used, which functions are prioritized, and whether antibodies rely on single or multiple Fc modifications. By mining curated entries in IMGT/mAb-DB, this study steps back to examine Fc engineering trends across approved and clinical-stage molecules. The review highlights: Fc engineering is widespread across therapeutic antibodies and fusion proteins cataloged in IMGT/mAb-DB Effector silencing strategies are commonly employed, particularly in programs prioritizing safety and controlled immune engagement Many molecules incorporate multiple Fc variants, rather than a single engineered change Fc modifications are used across a range of mechanisms and formats, underscoring Fc’s role as an intentional design lever

The Fc Review: Continuing our series taking a closer look at recent Fc-focused papers, what they found, and why it matters for antibody discovery and development. Can we program the Fc region? A recent bioRxiv preprint explores this question at scale, using millions of Fc variants to train machine learning models that predict functional outcomes across FcγR interactions. Background: Through engagement with Fc-receptors, the antibody Fc domain can direct a broad range of immune activities, including phagocytosis, cytokine release, antigen presentation, and immune cell polarization – each of which could be precisely tuned to combat disease. Fc engineering has traditionally focused on modifying one property at a time (E.g., ADCC, ADCP, or half-life). This work instead treats the Fc region as a functional design space and explores how sequence variations across the Fc domain can be linked to real immune engagement.

The Fc Review: One Fc variant, three advantages? A Nature Communications study demonstrates how a single Fc-engineered IgG achieved improved half-life, mucosal distribution, and enhanced immune-mediated killing, across both cancer and bacterial models. Background: Fc engineering is often discussed through a single lens, half-life extension, effector boosting, or silencing. This paper explores a broader question: can an Fc variant containing three point mutations deliver multiple functional gains across different biological systems?

The Fc Review: How does Fc engineering shape bispecific antibody function? A Frontiers in Bioengineering and Biotechnology review explores how the Fc region can be tuned to control effector function, half-life, and safety, key levers in the design of next-generation bispecific antibodies ( bsAbs ). Background: Bispecific antibodies bring new therapeutic possibilities by engaging multiple targets at once. But this complexity also brings new challenges, from unwanted immune activation to altered pharmacokinetics . The Fc region plays a central role here, acting as both a stabilizing scaffold and a regulator of immune effector engagement

The Fc Review: Continuing our series taking a closer look at recent Fc-focused papers, what they found, and why it matters for antibody discovery and development. How does Fc engineering shape the translation of antibodies from preclinical models to the clinic? A new industry-wide review with 15 case studies examines the impact of Fc modifications on pharmacology and safety, and the challenges of predicting human outcomes from nonclinical studies. Background: Fc regions do not only extend half-life, they drive functions like ADCC , ADCP, CDC, and immune modulation. Engineering the Fc can enhance, silence, or redirect these activities. But the same changes that deliver potency can also introduce risk, especially when preclinical models do not fully mirror human Fc receptor biology.
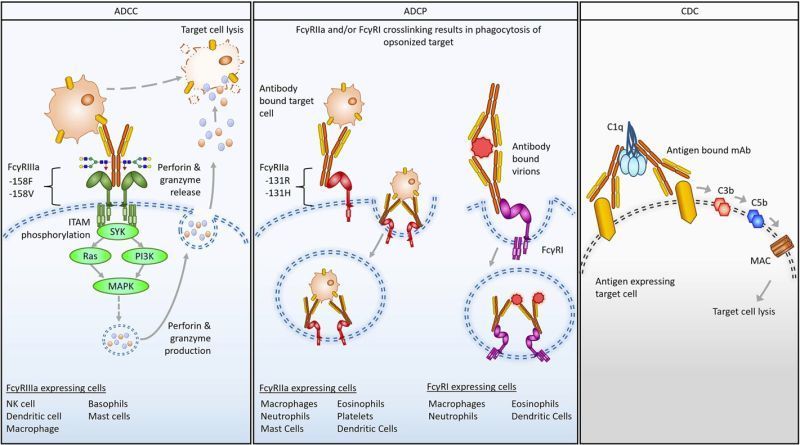
The Fc Review: Kicking off a new series where we take a closer look at recent Fc-focused papers. What they found, and why it matters for antibody discovery and development. How much does FcγR genetic variation influence an antibody’s function? A recent FDA review examines the often-overlooked role of Fcγ receptor (FcγR) polymorphisms in shaping therapeutic antibody activity, and the implications for the assays used to measure it. Background: FcγRs are the “effector arm” connection between antibodies and immune cells, driving processes like ADCC and ADCP. Genetic variation in these receptors can alter binding strength, modulate effector function, and impact clinical outcomes. Understanding this interplay is important for therapeutic design, potency assessment, and patient response prediction.


