Design and engineering of bispecific antibodies: insights and practical considerations
The Fc Review:
How does Fc engineering shape bispecific antibody function?
A Frontiers in Bioengineering and Biotechnology review explores how the Fc region can be tuned to control effector function, half-life, and safety, key levers in the design of next-generation bispecific antibodies (bsAbs).
Background:
Bispecific antibodies bring new therapeutic possibilities by engaging multiple targets at once. But this complexity also brings new challenges, from unwanted immune activation to altered pharmacokinetics. The Fc region plays a central role here, acting as both a stabilizing scaffold and a regulator of immune effector engagement
The review highlights:
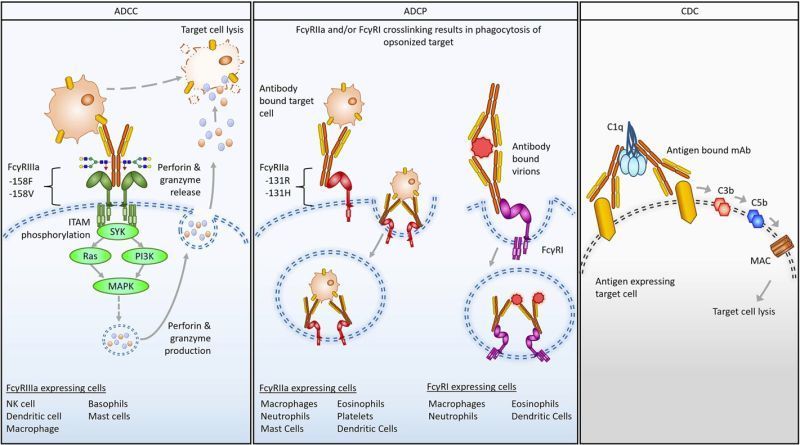
- Fc engineering and glycoengineering can enhance or silence functions like
ADCC and
ADCP.
- FcRn affinity tuning improves antibody half-life and tissue distribution.
- Tailoring Fc interactions enables bispecifics with new modes of action, including targeted agonism and dual checkpoint blockade.
Implications:
Thoughtful Fc design is essential for achieving the right balance between potency, pharmacokinetics, and safety in bispecific development.
Our perspective:
This work underscores how small Fc changes can have big impacts on antibody function and therapeutic performance. At SeromYx, our platform is built to capture those effects, using primary-cell assays and multiplexed Fc profiling to reveal how engineered antibodies actually engage human effector pathways. For teams advancing bispecifics, that means earlier insight into how design choices translate to real biological outcomes.