An Fc-Engineered Glycomodified Antibody Supports Proinflammatory Activation of Immune Effector Cells and Restricts Progression of Breast Cancer
Abstract:
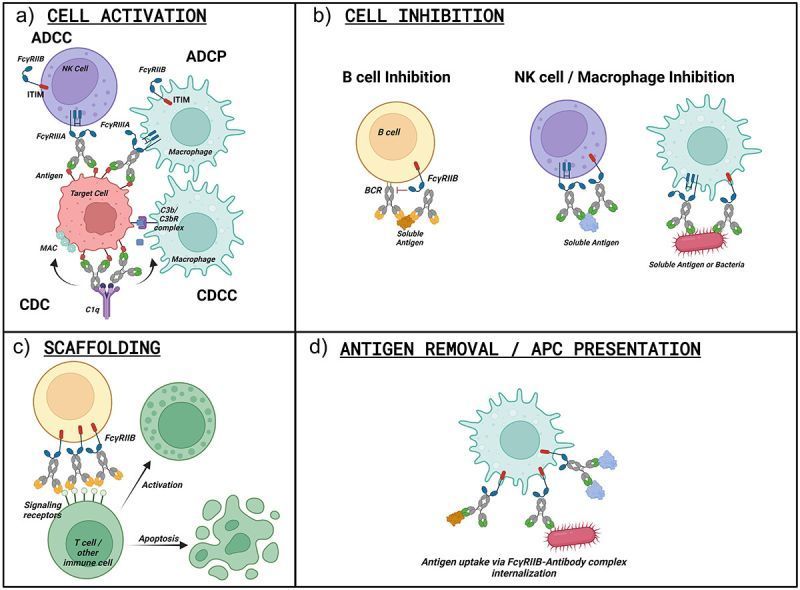
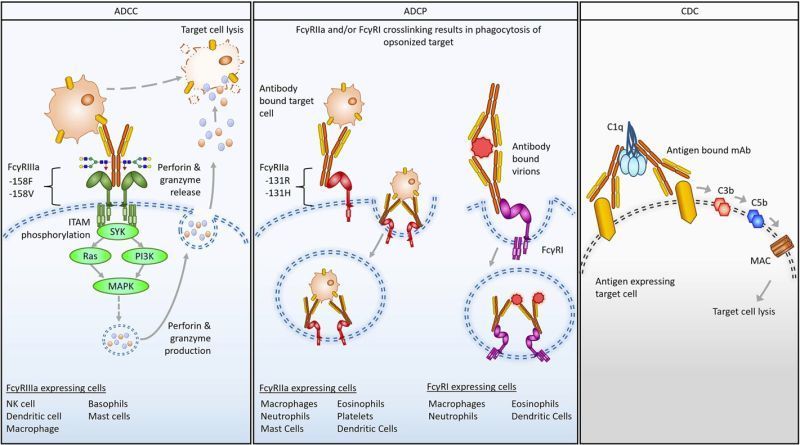
Fc engineering to enhance antibody effector functions harbors the potential to improve therapeutic effects. Understanding FcγR expression and distribution in the tumor microenvironment prior to and following treatment may help guide immune-engaging antibody design and patient stratification. In this study, we investigated FcR-expressing immune effector cells in HER2+ and triple-negative breast cancers (TNBC), including neoadjuvant chemotherapy–resistant disease. FcγRIIIa expression, FcγRIIIa+ NK cells, and classically activated (M1-like) macrophages correlated with improved anti-HER2 antibody efficacy. FcγRIIIa protein and FcγRIIIa+ NK cells and macrophages were present in primary TNBC and retained in treatment-resistant tumors.
FcγRIIIa was spatially associated with folate receptor alpha–positive (FRα+) tumor areas at baseline and in residual tumors following neoadjuvant chemotherapy. Wild-type and Fc-engineered antibodies recognizing two breast cancer–associated antigens, HER2 and the emerging TNBC target FRα, were designed and generated to have increased FcγRIIIa-expressing effector cell engagement. The combination of glycoengineering, including fucose removal from the N-linked Fc glycan, and Fc point mutations greatly increased antibody affinity for and retention on FcγRIIIa. The Fc-engineered antibodies enhanced immune effector activity against HER2+ breast cancer and TNBC, altering proinflammatory cytokine production by NK cells and tumor-conditioned macrophages and skewing macrophages toward proinflammatory states. Furthermore, the Fc-engineered antibodies restricted orthotopic HER2+ and FRα+ breast cancer xenograft growth at doses suboptimal for equivalent wild-type antibodies and recruited FcγRIIIa-expressing cells into tumors. Antibody design through combined glycoengineering and Fc point mutations to enhance FcγRIIIa engagement of tumor-infiltrating effector cells may be a promising strategy for developing therapies for patients with aggressive and treatment-resistant breast cancers.
Significance:
Assessment of Fc receptors and immune cells in breast cancer enables development of tailored engineering strategies for tumor-targeting monoclonal antibodies with enhanced immune-stimulating and anticancer attributes by combining glycoengineering and Fc mutations.
Authors:
Chenoweth, A. M., et al. (2025). An Fc-engineered glycomodified antibody supports proinflammatory activation of immune effector cells and restricts progression of breast cancer. Cancer Research.
Journal:
Cancer Research