New antibody restricts the growth of aggressive and treatment-resistant breast cancers
New antibody restricts the growth of aggressive and treatment-resistant breast cancers
A new potential antibody therapy strategy which restricts the growth of treatment-resistant breast cancers has been developed by scientists.
The King’s College London discovery, published today, could provide new treatment options for some of the most aggressive forms of breast cancer. This may be particularly important for patients whose cancers no longer respond to existing therapies, as well as those with triple-negative breast cancer – a subtype which lacks the receptors which are common drug targets, where treatment choices remain very limited.
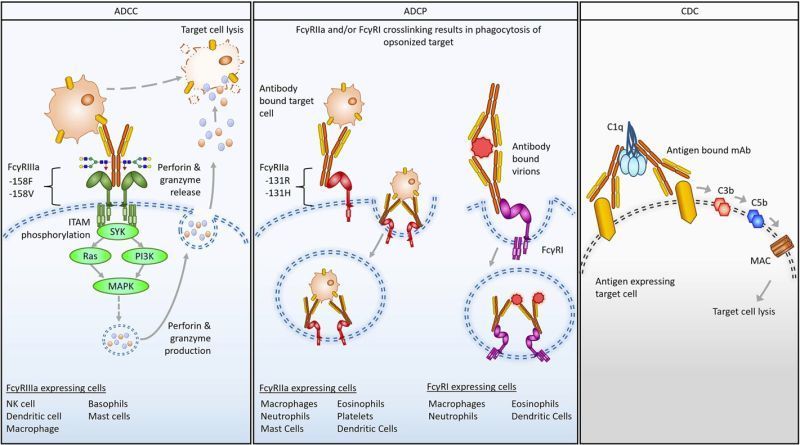
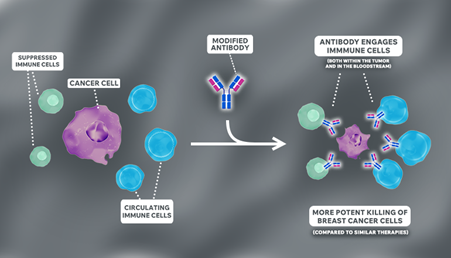
The team designed an antibody that not only attacks the tumour cells directly, but also harnesses the body’s own immune defences. The first of its kind ‘triple-engineered antibody’, latches onto cancer cells on one end and draws in immune cells on the other.
While researchers have been modifying antibodies to boost their ability to activate immune cells, highly effective ones to be used for the treatment of breast cancer are still needed. The Breast Cancer Now Research Unit at King’s College London has been at forefront of this research for more than a decade. The group focuses on studying the patient’s immune system with a view of designing and testing innovative antibodies able to activate the patient’s immune response.
In their latest study, laboratory experiments and animal models revealed the modified antibody bound immune cells more strongly compared to current treatments. This activated the immune cells already present in the tumour to attack it, limiting the growth of tumours in triple-negative and treatment-resistant breast cancers.
The researchers also found that the modified antibody activated immune cells circulating in the bloodstream, which could boost the body’s overall ability to detect and fight cancer.
First author Dr Alicia Chenoweth, from the Faculty of Life Sciences & Medicine at King’s College London, said: “By making a few key changes in the structure of the antibody, we found that it could activate the immune system much more powerfully than an unmodified antibody currently used in breast cancer treatment.
“Many of the immune cells in breast tumours are in a ‘suppressed’ state, difficult to activate with unmodified antibodies. We found our triple-engineered antibodies were not only able to activate these immune cells to kill the cancer cells, but shifted these immune cells to a more ‘activated’ state overall.”
Lead author Professor Sophia Karagiannis, expert in Translational Cancer Immunology and Immunotherapy at King’s College London, who led this study said: “By examining key immune cell receptors in breast tumours, including those tumours resistant to chemotherapy and immunotherapy, we have designed our antibody to make them interact better and harness the immune system in a way that has never been done or tested in cancer before.
“If it proves successful, it could stimulate the immune system directly and address the significant unmet need we see in treatment resistant cancers including triple-negative breast cancer.”
Triple-negative breast cancer accounts for around 15 per cent of all breast cancers. It is a subtype that lacks receptors for the hormones oestrogen and progesterone and the HER2 protein, which are often treatment targets in other subtypes of breast cancer. As it lacks these targets, standard hormone therapies and drugs that target HER2 are ineffective, leaving patients with fewer treatment options and a higher risk of recurrence.
HER2-positive cancers are often treated with drugs, which improve outcomes for many patients, but the development of resistance remains a clinical challenge.
Dr Simon Vincent, chief scientific officer at Breast Cancer Now, said: “This promising, early-stage research offers hope for more and better treatments for over 8,000 women who are diagnosed with triple negative breast cancer each year in the UK.
“We know how urgently these women need new treatment options, as this form of the disease can be more challenging to treat, may be more likely to return or spread in the first few years following treatment, and it affects younger women and black women more than other groups. By funding research like this, we’re driving progress towards ensuring everyone with breast cancer lives and lives well.”
The team is now working towards developing immune-active antibodies so that one day these can be tested in patients in clinical trials. Further laboratory work is underway to optimise the therapy, including extending how long the antibody lasts in the body and ensuring it can activate a broader range of immune cell types.
The research not only highlights the potential of this therapy for breast cancer patients but could be used in other cancers. One of the antibody targets is also present in ovarian and endometrial cancers, so the approach might have clinical potential for these diseases too.
The study was published in Cancer Research, a journal of the American Association for Cancer Research
Notes to Editors
Funding:
- Asda Tickled Pink fully funds Breast Cancer Now’s research unit at King’s College London, contributing over £1 million annually to advance knowledge of triple negative breast cancer - one of the hardest types to treat.
Industrial Partners:
- SeromYx Systems, Inc., 299 Washington St, Ste D, Woburn, MA 01801 USA
- Ludger, Ltd., Culham Campus, Abingdon, Oxfordshire OX14 3EB, United Kingdom;