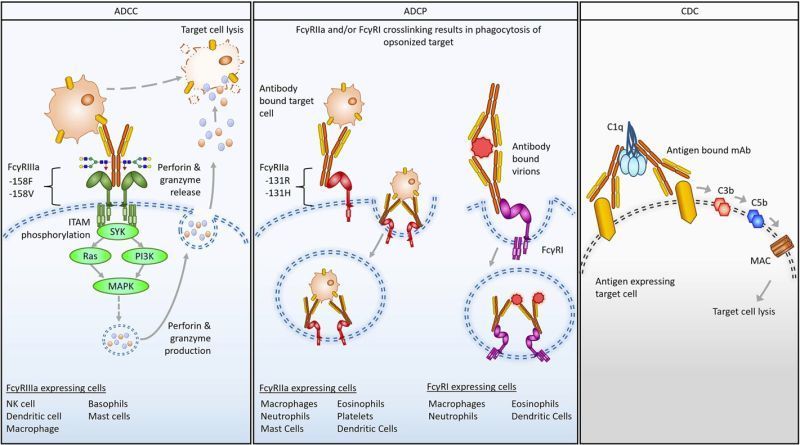
Fc engineering by monoclonal mammalian cell display for improved affinity and selectivity towards FcγRs
The Fc Review:
How are antibody developers actually using Fc engineering today?
A recent analysis of the IMGT/mAb-DB database takes a systematic look at engineered Fc variants across therapeutic antibodies and fusion proteins, offering a real-world snapshot of how Fc design choices are being deployed in the clinic.
Background:
Fc engineering is often discussed in terms of individual mutations or isolated use cases. But at an industry level, it’s less clear how frequently Fc variants are used, which functions are prioritized, and whether antibodies rely on single or multiple Fc modifications. By mining curated entries in IMGT/mAb-DB, this study steps back to examine Fc engineering trends across approved and clinical-stage molecules.
The review highlights:
- Fc engineering is widespread across therapeutic antibodies and fusion proteins cataloged in IMGT/mAb-DB
- Effector silencing strategies are commonly employed, particularly in programs prioritizing safety and controlled immune engagement
- Many molecules incorporate multiple Fc variants, rather than a single engineered change
- Fc modifications are used across a range of mechanisms and formats, underscoring Fc’s role as an intentional design lever

Figure 2. Fc engineering by monoclonal mammalian cell display. (A) Schematic of the process. Fc library plasmids carrying error-prone mutagenesis at the hinge-CH2 region were introduced to RMCE ready CHO cells via aRMCE [34]. Transfected cells were incubated with Fcγ R bound beads for MACS to isolate IgG displaying cells. Post-MACS cells were screened by two rounds of FACS for the higher
affinity towards Fcγ RIIIa-F176, Fcγ RIIa, and Fcγ RIIb, respectively. (B) Ratiometric flow cytometry analysis of Fc WT and known variants with antigen EGFR-TNFα, individual Fcγ R-biotin, and SA-APC. (C) Ratiometric flow cytometry analysis of library cells during the screening procedures.
Implications for antibody development:
These data suggest that Fc engineering is no longer an exception, but a norm. As antibody programs increasingly rely on combinations of Fc mutations to tune activity, safety, and/or exposure, understanding how those changes behave together within a single Fc domain becomes critical. Developing Fc mutations in isolation and later combining them assumes additive behavior, an assumption that may not always hold and can lead to unexpected functional outcomes.
Even single, well-established Fc modifications can have effects beyond what they were introduced to achieve, highlighting the importance of evaluating Fc changes more broadly.
Our perspective:
As Fc design becomes more complex and purposeful, developers may benefit from evaluating engineered antibodies across broader functional contexts. Profiling Fc behavior across multiple receptors and immune pathways can help clarify how combined Fc variants translate into real biological outcomes, particularly early in development when design decisions are still flexible.
References:
Giudicelli V. et al. Identification of engineered IMGT Fc variants in IMGT/mAb-DB, a database of therapeutic antibodies and fusion proteins. mAbs, 2025. https://doi.org/xxxxx