Analysis of Fc-dependent effector functions of anti-malaria circumsporozoite protein antibodies
Journal Abstract:
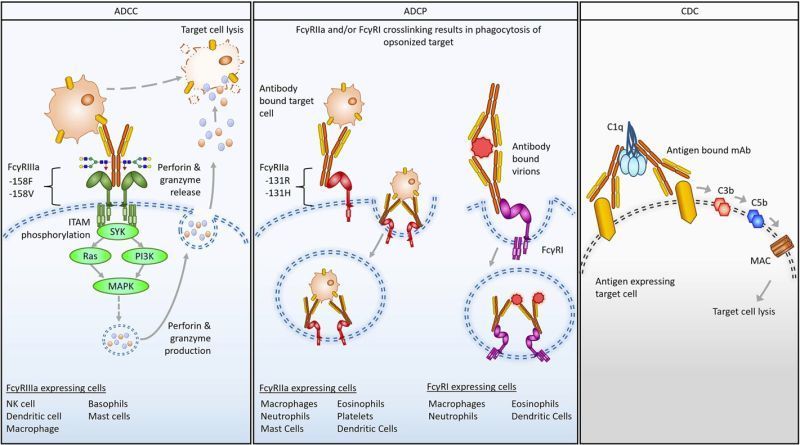
Antibodies targeting the malaria circumsporozoite protein (CSP) can prophylactically protect against malaria by targeting Plasmodium parasites before they establish symptomatic blood-stage disease. Engineering the antibody Fc region to more effectively engage immune effector functions has produced therapeutic antibodies with enhanced potency against viral and oncological targets. However, whether Fc-dependent immune effector functions can contribute to the protection of malaria CSP mAbs or be further enhanced via engineering has been limitedly tested. Here, we report that Fc-dependent effector functions are required for achieving maximal protection via prophylactic treatment with the CSP mAb 317. We further report that Fc engineering modulated the activity of multiple CSP mAbs in multiple in vitro assays of effector function. Our studies revealed that the mAbs L9 and CIS43 were more potent drivers of antibody-dependent phagocytosis, NK activation and killing, and complement deposition. In contrast, 317, but not L9 and CIS43, drove enhanced activation of CSP-responsive T-cells after DC acquisition of mAb-complexed antigens. Collectively, our data suggest that effector function represents an important mechanism of anti-CSP antibodies with the potential to enhance activity through Fc engineering.
Authors:
Stefanutti E, Ramani R, Whitener B, Dang H, Bélanger S, Somasundaram L, Cortina K, De Marco A, Tam T, Chai Q, Cameroni E, Gupta R, Schmid MA, Miller JL, Zumsteg AB, Purcell LA, Drewry LL. 0. Analysis of Fc-dependent effector functions of anti-malaria circumsporozoite protein antibodies. Microbiol Spectr 0:e00863-25.
Journal:
Microbiology Spectrum