mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions
Authors:
Kaplonek P, et al. Sci Transl Med. 2022 May 18;14(645):eabm2311.
Journal:
Science Translational Medicine
Journal Abstract/ Summary:
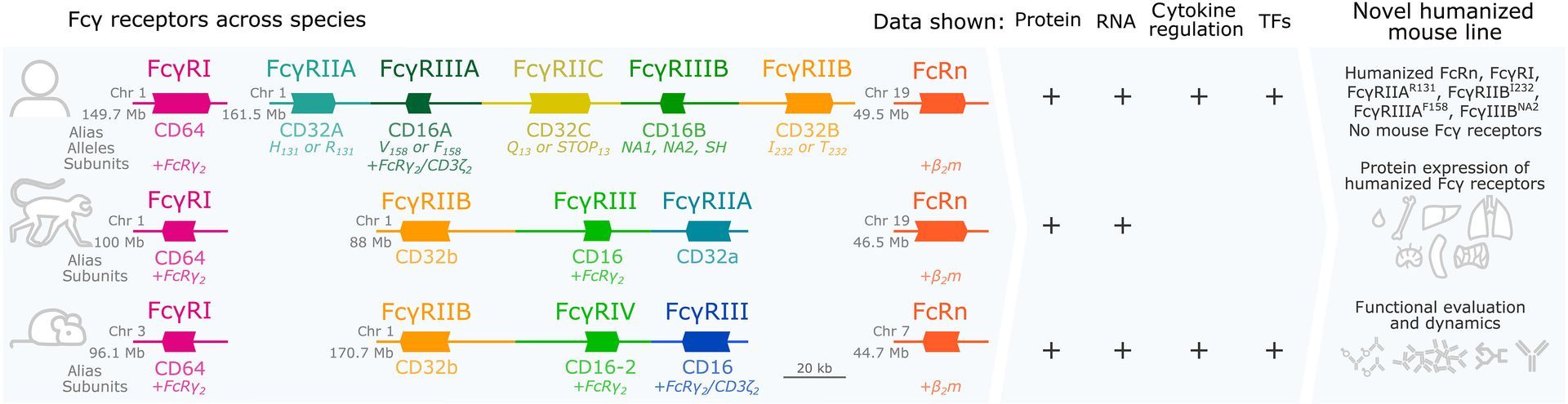
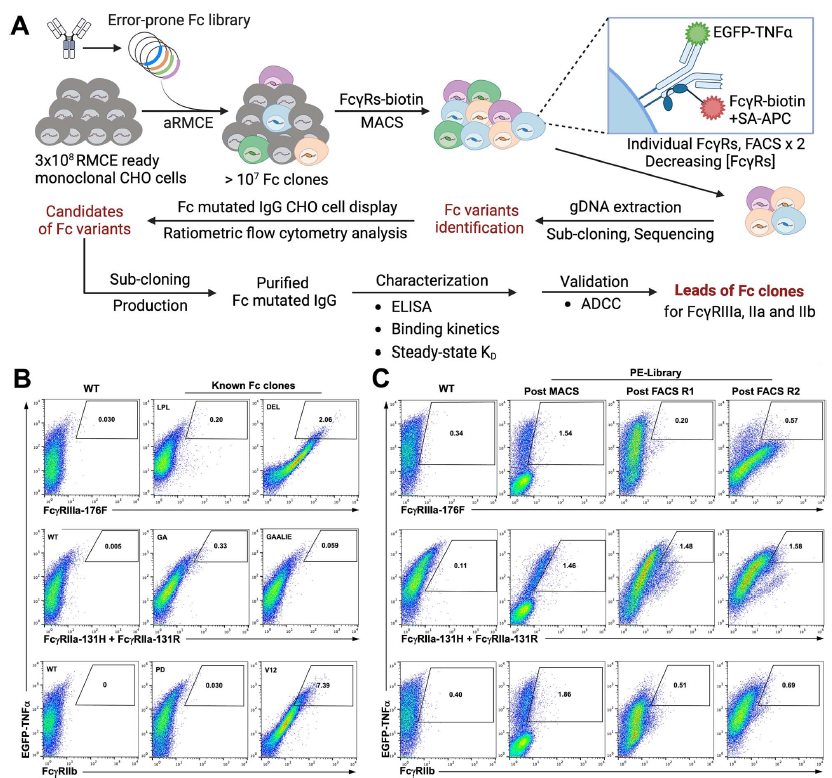
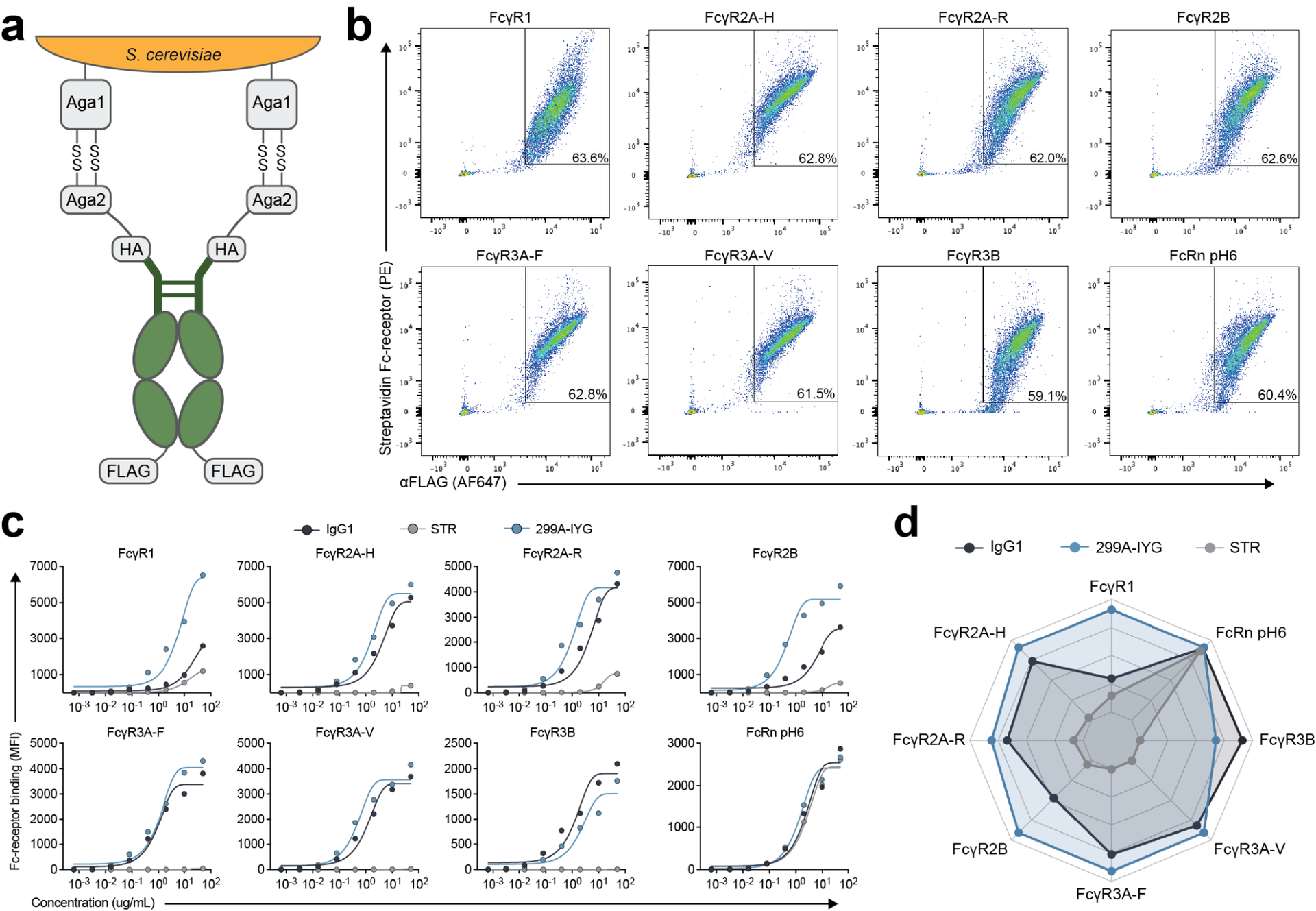
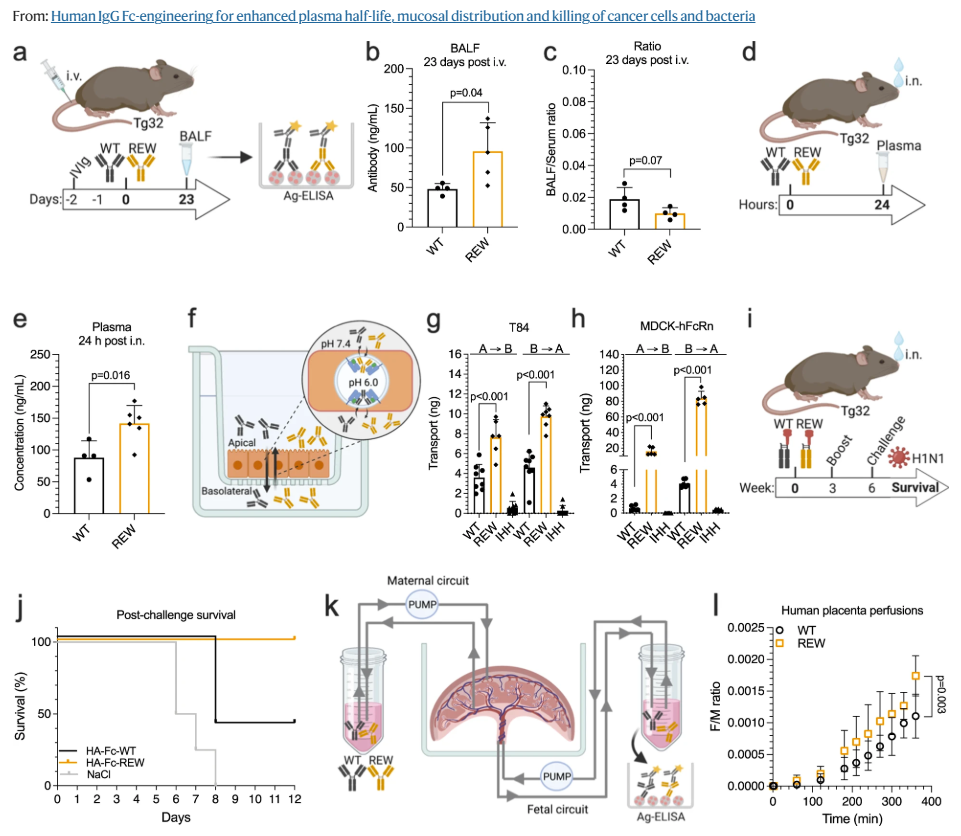
The successful development of several coronavirus disease 2019 (COVID-19) vaccines has substantially reduced morbidity and mortality in regions of the world where the vaccines have been deployed. However, in the wake of the emergence of viral variants that are able to evade vaccine-induced neutralizing antibodies, real-world vaccine efficacy has begun to show differences across the two approved mRNA platforms, BNT162b2 and mRNA-1273; these findings suggest that subtle variation in immune responses induced by the BNT162b2 and mRNA-1273 vaccines may confer differential protection. Given our emerging appreciation for the importance of additional antibody functions beyond neutralization, we profiled the postboost binding and functional capacity of humoral immune responses induced by the BNT162b2 and mRNA-1273 vaccines in a cohort of hospital staff. Both vaccines induced robust humoral immune responses to wild-type severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and to variants of concern. However, differences emerged across epitope-specific responses, with higher concentrations of receptor binding domain (RBD)- and N-terminal domain-specific IgA observed in recipients of mRNA-1273. Antibodies eliciting neutrophil phagocytosis and natural killer cell activation were also increased in mRNA-1273 vaccine recipients as compared to BNT162b2 recipients. RBD-specific antibody depletion highlighted the different roles of non-RBD-specific antibody effector functions induced across the mRNA vaccines. These data provide insights into potential differences in protective immunity conferred by these vaccines.
Download PDF: https://pubmed.ncbi.nlm.nih.gov/35348368/