Evaluation of strategies to modify Anti-SARS-CoV-2 monoclonal antibodies for optimal functionality as therapeutics
Journal Abstract:
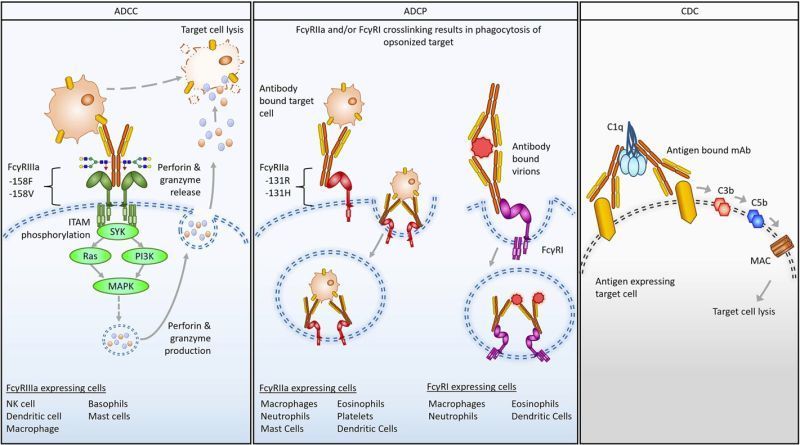
The current global COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a public health crisis with more than 168 million cases reported globally and more than 4.5 million deaths at the time of writing. In addition to the direct impact of the disease, the economic impact has been significant as public health measures to contain or reduce the spread have led to country wide lockdowns resulting in near closure of many sectors of the economy. Antibodies are a principal determinant of the humoral immune response to COVID-19 infections and may have the potential to reduce disease and spread of the virus. The development of monoclonal antibodies (mAbs) represents a therapeutic option that can be produced at large quantity and high quality. In the present study, a mAb combination mixture therapy was investigated for its capability to specifically neutralize SARS-CoV-2. We demonstrate that each of the antibodies bind the spike protein and neutralize the virus, preventing it from infecting cells in an in vitro cell-based assay, including multiple viral variants that are currently circulating in the human population. In addition, we investigated the effects of two different mutations in the Fc portion (YTE and LALA) of the antibody on Fc effector function and the ability to alleviate potential antibody-dependent enhancement of disease. These data demonstrate the potential of a combination of two mAbs that target two different epitopes on the SARS-CoV2 spike protein to provide protection against SARS-CoV-2 infection in humans while extending serum half-life and preventing antibody-dependent enhancement of disease.
Authors:
House, R. V., Broge, T. A., Suscovich, T. J., Snow, D. M., Tomic, M. T., Nonet, G., … Earnhart, C. G. (2022). Evaluation of strategies to modify Anti‑SARS‑CoV‑2 monoclonal antibodies for optimal functionality as therapeutics. PLOS ONE, 17(6), e0267796.
Journal:
PLOS One