View from the Fc: Five Rules for mAb Development Risk Reduction
Summary:
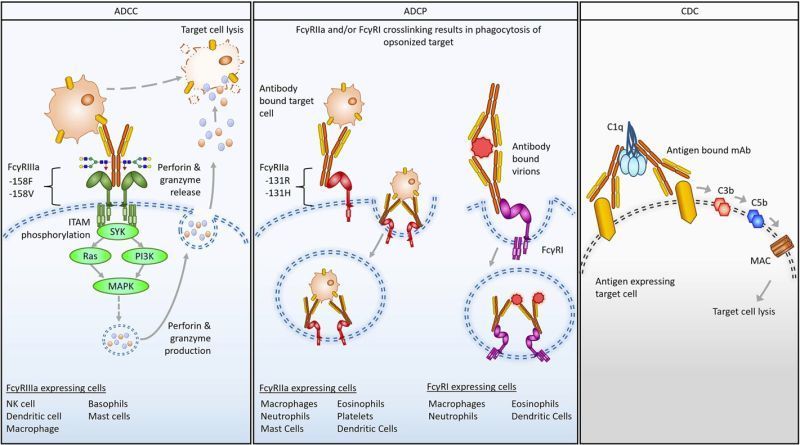
Designing monoclonal antibodies and related modalities such as ADCs and bispecifics with appropriate Fc function is critical to product safety and efficacy, but fiendishly hard. Outcomes are contextual, meaning that what works in one disease state/epitope setting may not hold in another, so the “rules” keep changing. And the factors driving Fc function are strongly interrelated, meaning that changes with one intent often have unexpected and undesirable consequences elsewhere. SeromYx offers the broadest and robust platform for Fc functional characterization.
Where does our platform fit in? We provide a unique resource for antibody discovery and development with an industrial quality system. While we add value throughout the mAb discovery and development process, from pre-IND to BLA enabling, we encourage developers to utilize our capabilities earlier so that the best candidates can be identified and nominated for the clinic. By measuring outcomes in a robust way, we offer a distinct advantage to developers so that they can maximize the rewards of approvals and minimize the risk of clinical failures due to underexploited and/or incompletely characterized Fc biology.