Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection
Introduction:
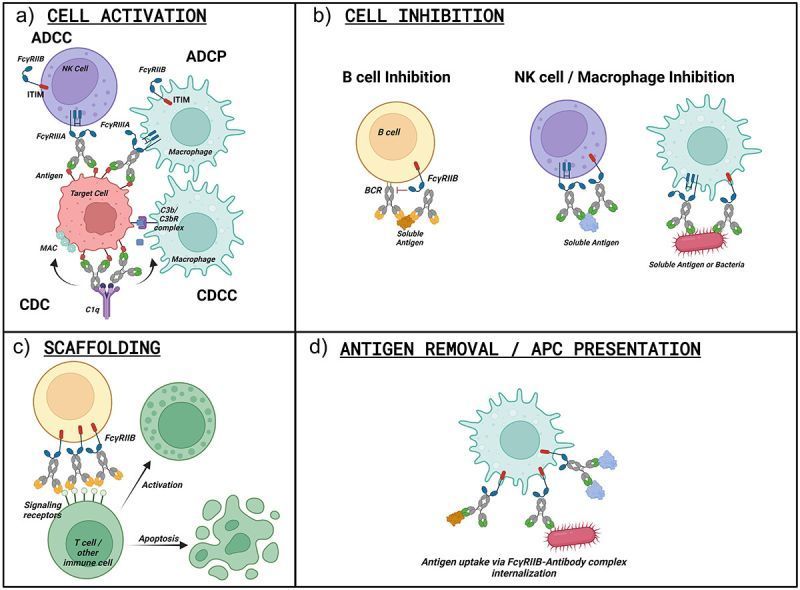
Nirsevimab is an extended half-life (M252Y/S254T/T256E [YTE]-modified) monoclonal antibody to the pre-fusion conformation of the respiratory syncytial virus (RSV) Fusion protein, with established efficacy in preventing RSV-associated lower respiratory tract infection in infants for the duration of a typical RSV season. Previous studies suggest that nirsevimab confers protection via direct virus neutralization. Here we use preclinical models to explore whether fragment crystallizable (Fc)-mediated effector functions contribute to nirsevimab-mediated protection.
Methods:
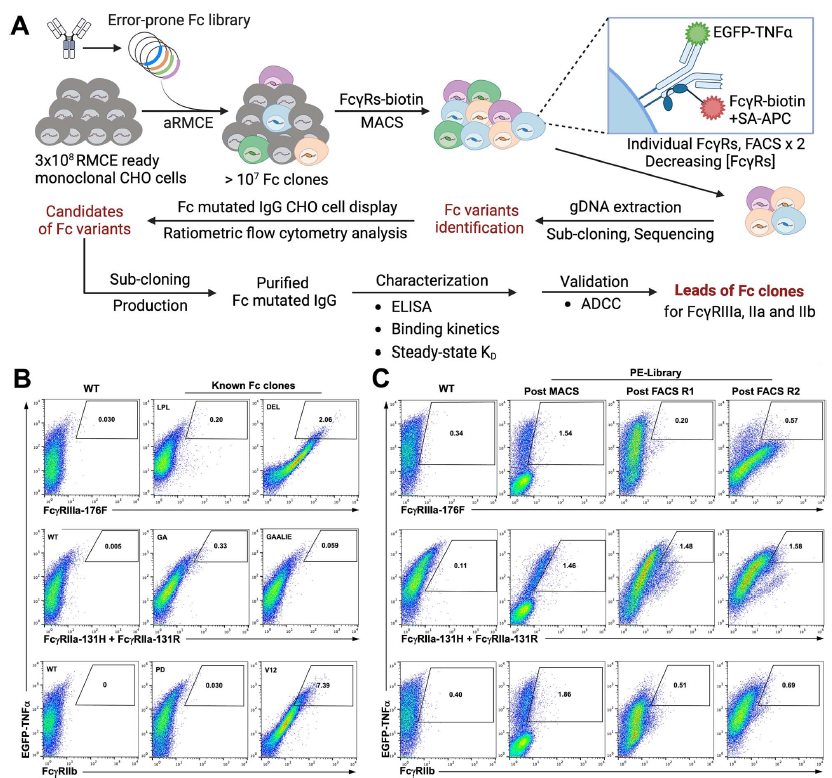
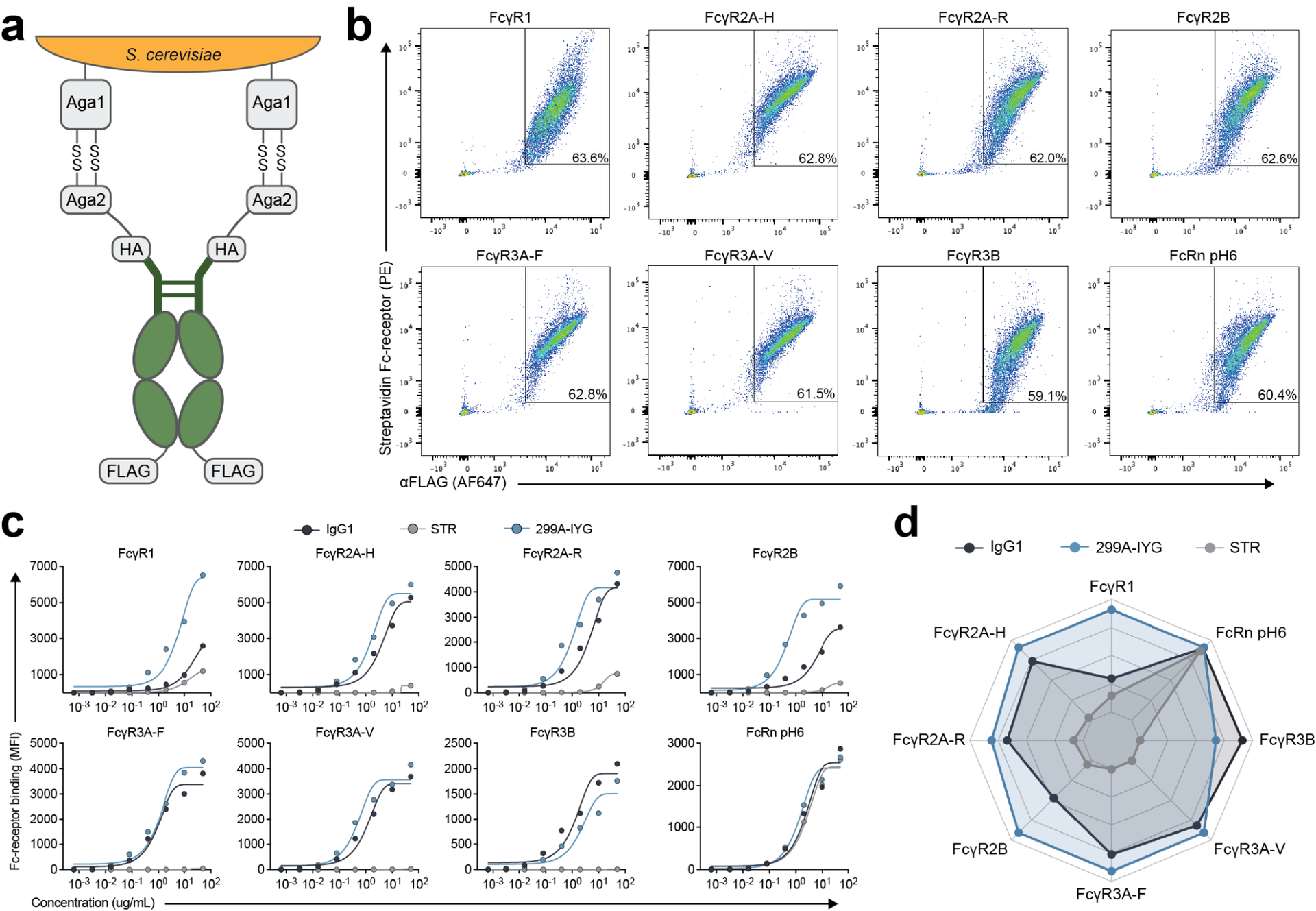
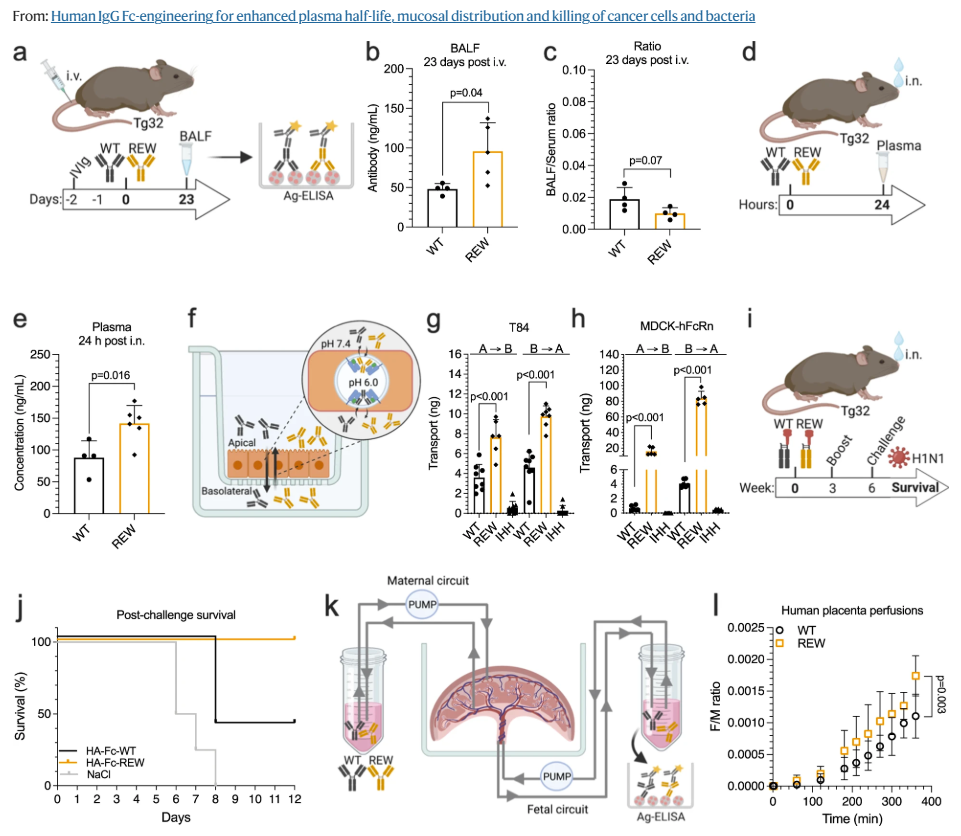
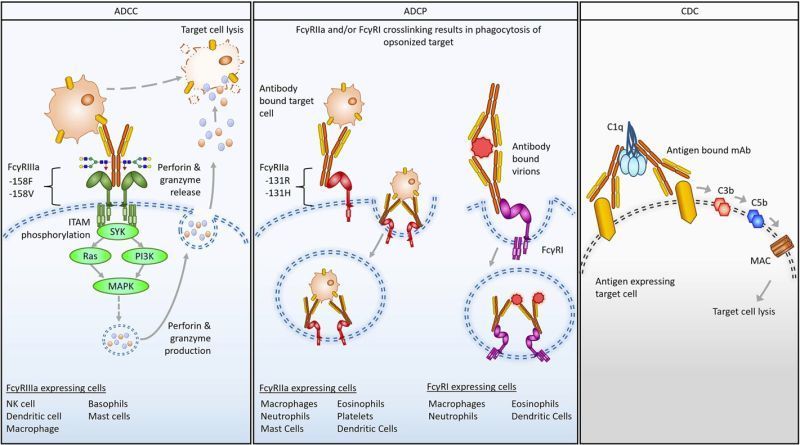
Nirsevimab, MEDI8897* (i.e., nirsevimab without the YTE modification), and MEDI8897*-TM (i.e., MEDI8897* without Fc effector functions) binding to Fc γ receptors (FcγRs) was evaluated using surface plasmon resonance. Antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent cellular phagocytosis (ADCP), antibody-dependent complement deposition (ADCD), and antibody-dependent cellular cytotoxicity (ADCC) were assessed through in vitro and ex vivo serological analyses. A cotton rat challenge study was performed with MEDI8897* and MEDI8897*-TM to explore whether Fc effector functions contribute to protection from RSV.
Results:
Nirsevimab and MEDI8897* exhibited binding to a range of FcγRs, with expected reductions in FcγR binding affinities observed for MEDI8897*-TM. Nirsevimab exhibited in vitro ADNP, ADCP, ADCD, and ADCC activity above background levels, and similar ADNP, ADCP, and ADCD activity to palivizumab. Nirsevimab administration increased ex vivo ADNP, ADCP, and ADCD activity in participant serum from the MELODY study (NCT03979313). However, ADCC levels remained similar between nirsevimab and placebo. MEDI8897* and MEDI8897*-TM exhibited similar dose-dependent reduction in lung and nasal turbinate RSV titers in the cotton rat model.
Conclusion:
Nirsevimab possesses Fc effector activity comparable with the current standard of care, palivizumab. However, despite possessing the capacity for Fc effector activity, data from RSV challenge experiments illustrate that nirsevimab-mediated protection is primarily dependent on direct virus neutralization.
Authors:
Brady, Tyler et al. Frontiers in Immunology, Volume 14, 1283120.
Journal:
Frontiers in Immunology