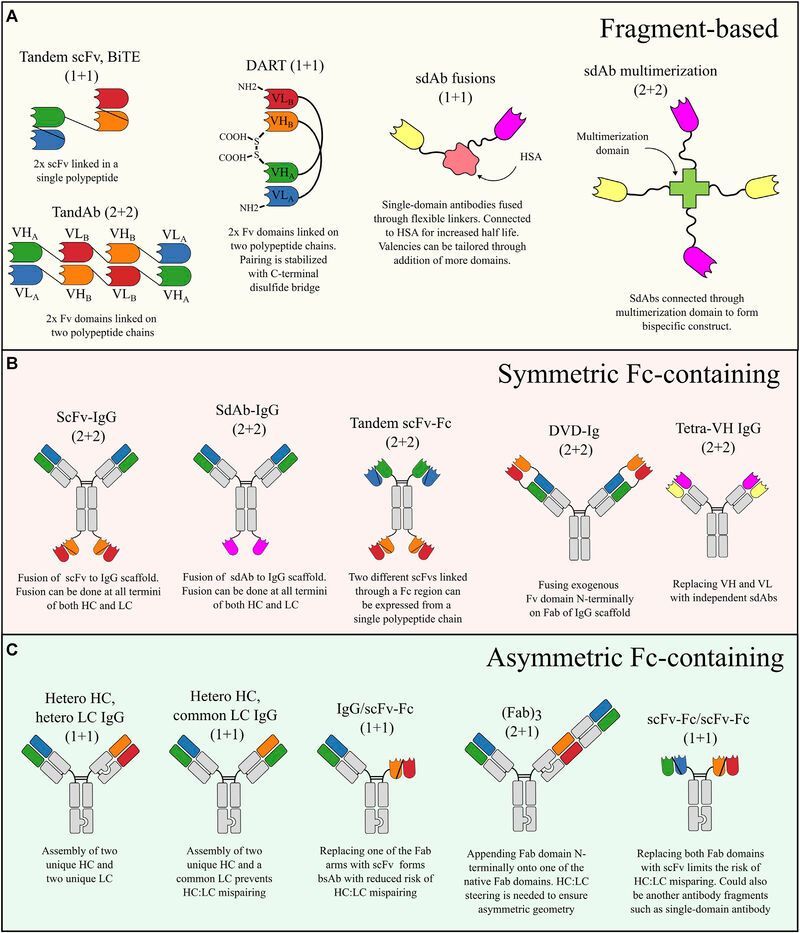
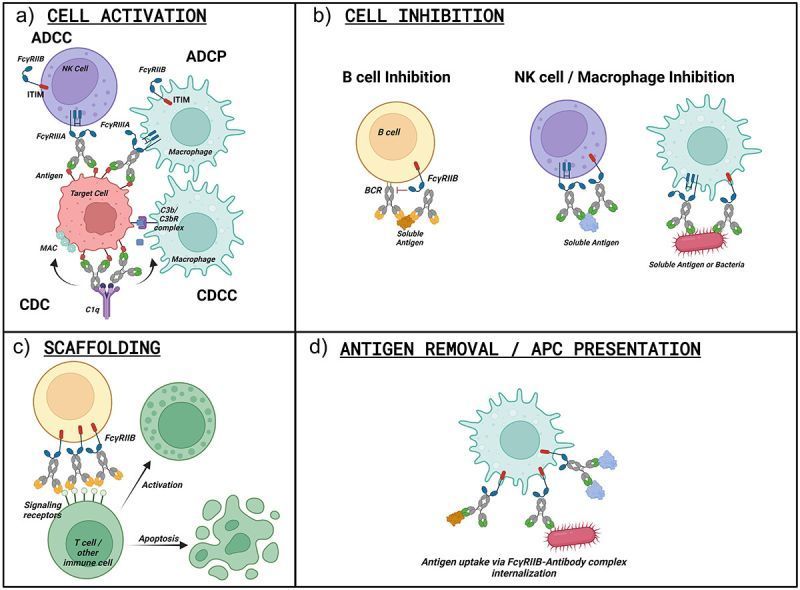
Systems serology for evaluation of HIV vaccine trials
Authors:
Ackerman ME, Barouch DH, Alter G. Systems serology for evaluation of HIV vaccine trials. Immunol Rev. 2017 Jan;275(1):262-270. doi: 10.1111/imr.12503. PMID: 28133810; PMCID: PMC5292205.
Journal:
Immunological Reviews
Journal Abstract/ Summary: The scale and scope of the global epidemic, coupled to challenges with traditional vaccine development approaches, point toward a need for novel methodologies for HIV vaccine research. While the development of vaccines able to induce broadly neutralizing antibodies remains the ultimate goal, to date, vaccines continue to fail to induce these rare humoral immune responses. Conversely, growing evidence across vaccine platforms in both non-human primates and humans points to a role for polyclonal vaccine-induced antibody responses in protection from infection. These candidate vaccines, despite employing disparate viral vectors and immunization strategies, consistently identify a role for functional or non-traditional antibody activities as correlates of immunity. However, the precise mechanism(s) of action of these “binding” antibodies, their specific characteristics, and their ability to be selectively induced and/or potentiated to result in complete protection merits parallel investigation to neutralizing antibody-based vaccine design approaches. Ultimately, while neutralizing and functional antibody-based vaccine strategies need not be mutually exclusive, defining the specific characteristics of “protective” functional antibodies may provide a target immune profile to potentially induce more robust immunity against HIV. Specifically, one approach to guide the development of functional antibody-based vaccine strategies, termed “systems serology”, offers an unbiased and comprehensive approach to systematically survey humoral immune responses, capturing the array of functions and humoral response characteristics that may be induced following vaccination with high resolution. Coupled to machine learning tools, large datasets that explore the “antibody-ome” offer a means to step back from anticipated correlates and mechanisms of protection and toward a more fundamental understanding of coordinated aspects of humoral immune responses, to more globally differentiate among vaccine candidates, and most critically, to identify the features of humoral immunity that distinguish protective from non-protective responses. Overall, the systematic serological approach described here aimed at broadly capturing the enormous biodiversity in antibody profiles that may emerge following vaccination, complements the existing cutting edge tools in the cellular immunology space that survey vaccine-induced polyfunctional cellular activity by flow cytometry, transcriptional profiling, epigenetic, and metabolomic analysis to offer a means to develop both a more nuanced and a more complete understanding of correlates of protection to support the design of functional vaccine strategies.
Download PDF: https://onlinelibrary.wiley.com/doi/10.1111/imr.12503