5. Manufacturing
Leveraging Digital SPR to Differentiate Vaccine Manufacturing Processes
Integrating antibody avidity with Fc effector profiling to enhance vaccine assessment.
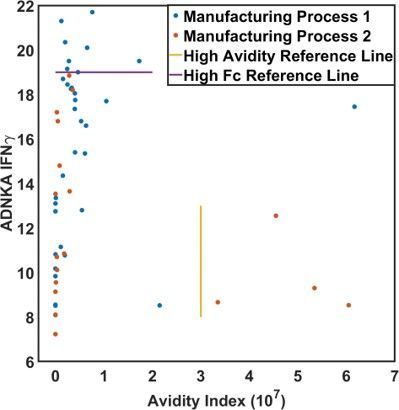
By combining Systems Serology with surface plasmon resonance (SPR)–based avidity measurements, this study uncovered distinct immune profiles differentiating two vaccine manufacturing processes. While one process drove higher effector function with lower avidity, the other generated stronger avidity with reduced effector activity. Multivariate modeling highlighted avidity, NK cell IFNγ responses, and IgG1 as key features distinguishing the processes, providing mechanistic insight into how manufacturing influences vaccine-induced immunity.
- Links antibody avidity with functional immune readouts
- Differentiates immune profiles across vaccine manufacturing processes
- Provides mechanistic insight to guide vaccine design and optimization


